Abstract
Free full text

CaV1.2 Calcium Channel Expression in Reactive Astrocytes is associated with the Formation of Amyloid-β Plaques in an Alzheimer’s Disease Mouse Model
Abstract
Increased activity of L-type Ca2+ channels has been implicated in the pathogenesis of dementia and Alzheimer’s disease (AD). Previously we detected CaV1.2 α1-subunit-positive expression in reactive astrocytes surrounding the plaques of 12 month-old transgenic mice overexpressing hAβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations. Here we examined whether increased CaV1.2 α1-subunit expression precedes plaque formation or is specifically associated with the increased amyloid-β (Aβ) load in the plaques. Quantitative RT-PCR expression profiling of all high voltage-gated Ca2+ channel subunits (α1, β, and α2δ) revealed no difference in the hippocampi of 2, 4, and 11 month-old wild type (wt) and transgenic (tg) mice. Immunohistochemistry demonstrated that expression of CaV1.2 α1-subunit, but not of the auxiliary β4 Ca2+ channel subunit, specifically associated with Aβ-positive plaques in brains of 11 month tg mice. No difference in CaV1.2 α1-subunit labeling was found in 2 and 4 month-old wt and tg mice prior to plaque formation. The CaV1.2 α1-subunit-positive cells in 11 month-old tg mice also labeled with GFAP, but not with the microglia marker Iba1. In contrast, GFAP-positive cells induced by injection of quinolinic acid did not reveal any CaV1.2 α1-subunit immunoreactivity. Together these results indicate that the expression of CaV1.2 α1-subunits in reactive astrocytes in the tg AD mouse model is related to the increased amyloid-β load in the plaques rather than caused by effects on gene regulation or mechanisms preceding the manifestation of AD as seen by plaque formation.
INTRODUCTION
Progressive impairment in memory and cognition is a key clinical feature of Alzheimer’s disease (AD). The disorder is morphologically characterized by extracellular deposition of amyloid-β (Aβ), intraneuronal tau pathology, synaptic loss, neuronal cell death, Aβ angiopathy, and inflammatory processes. Besides these hallmarks of AD, altered Ca2+ regulation is likely to play an important role in the pathology of AD [1]. One version of the Ca2+ hypothesis of the pathogenesis of dementia maintains that increased activity of L-type Ca2+ channels (LTCC) drives many of the other biomarkers in aging and AD brains.
LTCCs consist of a pore-forming α1 subunit (CaV1.1, −1.2, −1.3, or −1.4) and accessory α2δ and β subunits [2]. Whereas the α1 subunits contain the voltage-sensor and channel pore and determine the pharmacological properties, the accessory subunits regulate membrane expression and gating properties of the Ca2+ channels [3]. The mammalian brain expresses mainly two types of LTCCs, CaV1.2 and CaV1.3 [4, 5]. These voltage-gated Ca2+ channels represent a major mechanism for activity-induced Ca2+ entry into neurons and regulate multiple neuronal cell functions, including excitability, transcriptional regulation, and synaptic plasticity. Accordingly, aberrant activity of LTCCs and dysregulation of Ca2+ homeostasis may possibly be involved in AD pathology, including altered neuronal excitability [6] and neuronal communication [7].
Transgenic (tg) mice overexpressing human amyloid-β protein precursor (hAβPP) with mutations that predispose for familial AD provide potent models to test specific alterations associated with an upregulation of Aβ [8, 9]. While most of these tg mouse models do not develop the typical neuronal cell loss observed in AD, they manifest age-dependent memory impairments and cognitive deficits [8-10]. In a recent study, we demonstrated that CaV1.2 α1-subunits are highly expressed in reactive astrocytes of 12 month-old tg mice overexpressing hAβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations [11]. However, at present it is unclear whether this increased expression of the CaV1.2 α1-subunit is causally related to the pathogenesis of AD. Specifically, it is unknown whether similar changes in CaV1.2 α1-subunit expression occur in younger tg mice at a time when the disease is not yet clinically manifest. Moreover, it is important to determine whether the altered expression of CaV1.2 α1-subunits is due to a primary effect of the disease-causing hAβPP mutations on gene regulation or whether it is caused secondarily by a mechanism related to the appearance of Aβ plaques.
To address these questions, we investigated the transcriptional regulation and the expression of the CaV1.2 α1 and β4 subunit proteins in brains of 2, 4, and 11 month-old wild type (wt) and tg mice overexpressing hAβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations. The results presented in the current study demonstrate that Ca2+ channel mRNA expression was unaffected by the AβPP disease mutations and that increased CaV1.2 α1-subunit was exclusively found at late disease stages (11 months) where it was specifically associated with GFAP-positive astrocytes near the plaques. These findings are consistent with an effect on CaV1.2 α1-subunit expression in reactive astrocytes of the Aβ load in the vicinity of the plaques rather than with a function of CaV1.2 early in the pathogenesis of AD at the pre-plaque stage.
MATERIALS AND METHODS
Animals
Tg animals over-expressing human AβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations under the regulatory control of the neuron specific murine Thy-1 promoter (mThy-1-hAβPP751), heterozygous with respect to the transgene, on a C57BL/6 background were used [12]. The colony was sustained by crossing hAβPP751 overexpressing mice with C57BL/6 mice (Harlan Winkelman, Germany). Age-matched littermates were used as controls. All mice were housed according to standard animal care protocols, fed ad libitum with regular animal diet, and maintained in a pathogen-free environment in single ventilated cages at QPS Austria (Grambach, Austria). The transgenic status of each animal was confirmed by real time PCR of tail snips using specific primers and the appropriate hybridization probe. The age of the animals was 2, 4, or 11 month at sacrifice. In order to address the specificity of the β4 antibody, a functional β4 null model of two week old lethargic (lh) mice has been used [13]. Using a CaV1.2 KO mouse, we convincingly demonstrated the specificity of the CaV1.2 α1-subunit antibody [11]. To explore astrogliosis induced by an excitotoxic brain lesion, wt mice were exposed to stereotaxic application of quinolinic acid (QA), an NMDA receptor agonist. C57BL/6 mice received intrastriatal injection of 90 nmol QA under deep isoflurane inhalation anesthesia at the following coordinates to Bregma: anterior-posterior: 0.7 mm, lateral: 1.9 mm, ventral: −2.5 mm. The incisor bar was set at zero [14]. All animal experiments were approved by the Austrian Ministry of Science and Research and conformed to the Austrian guidelines on animal welfare and experimentation.
Tissue preparation and immunohistochemistry
The mice were anaesthetized, transcardially perfused with phosphate-buffered saline (PBS; 50 mM, pH 7.4), followed by a phosphate-buffered 4% paraformaldehyde solution. Brains were dissected, post-fixed for 60 min in the same fixative, incubated in PBS containing 20% sucrose, frozen in a CO2 stream, and stored at −80°C until further processing. Brains were cut into coronal sections of 40 μm with a cryostat Leica CM 1950. Immunohistochemistry was performed on free-floating sections as described previously [15]. Brain sections were washed 30 min with 0.1% Triton X-100/PBS (T-PBS), subsequently pre-treated 20 min with 20% methanol/1% H2O2/PBS and after thorough rinsing, sections were blocked with 20% horse serum/0.2% BSA/T-PBS. Immunoblocking (2 drops ad 2.5 ml PBS, Vector M.O.M., MKB-2213) was performed for 1 h to avoid unspecific anti-mouse secondary antibody binding to mouse tissue. Finally, sections were incubated for 2–3 days at 4°C with a primary antibody diluted in 0.2% BSA/T-PBS. Primary antibodies used for this study were: rabbit anti-CaV1.2 α1-subunit (α1 C) (1:2000, Sigma C1603), mouse anti-Aβ (1:250, Sigma A8978), mouse anti-β4 (1:500, monoclonal, Neuromab, Davis, CA, USA), chicken anti-GFAP (1:2000, Millipore AB5541), and rabbit anti-Iba1 (1:500, Wako 019-19741). After incubation, sections were washed in PBS and incubated with biotinylated secondary antibodies: α-rabbit (1:200, Vector V1011), α-mouse (1:200, Vector N0429), or α-chicken (1:200, Vector K0722). After 1 h of incubation, sections were washed with PBS and incubated with an avidin-biotin complex solution (ABC-Elite Vectastain reagent Vector Lab.) for another hour. After washing with 50 mM Tris-buffered saline, the signal was detected by using 0.5 mg/ml 3,3′-diaminobenzidine (DAB) including 0.003% H2O2 as a substrate in Tris-buffered saline for 3–8 min. The reaction was terminated by transferring the sections into T-PBS. Stained sections were rinsed in 10 mM PBS, pH 7.4, mounted onto gelatine/chromalaun-coated glass slides, dehydrated in an ascending ethanol series, cleared in butylacetate, and cover slipped with Entellan® (Merck, Darmstadt, Germany). Control experiments for all antibodies were conducted by omitting the primary antibody. Staining was visualized with an Olympus BX61 fluorescence microscope and pictures captured with Openlab software connected to an Apple computer.
For colocalization studies, fluorescence immunohistochemistry was performed similar as for chromogenic staining but without methanol pre-treatment. After incubation with the primary antibody, sections were washed in PBS and incubated with fluorescent Alexa-488 or −546 or −350 secondary antibodies (Invitrogen–Life tech, Vienna, Austria). After 1 h of incubation, sections were washed and mounted onto gelatin/chromalaun-coated glass slides and cover slipped with Vectashield mounting medium (Vector H-1000).
RNA isolation and quantitative Taqman-PCR
Taqman RT-PCR on RNA isolated from brain tissue was performed according to a previously developed protocol [16]. Wt and tg mice of different age (2, 4, and 11 months) were sacrificed as described above. Brain regions were dissected, transferred to cold Hanks-balanced salt solution (HBSS), and immediately transferred into RNA later RNA Stabilization Reagent (Qiagen, GmbH, Hilden, Germany). Tissue samples were disrupted by using a rotor-stator homogenizer (Ultraturrax T8, IKA, Staufen, Germany) and QiaShredder columns. Total RNA was extracted from homogenized brain tissue using the RNeasy Protect Mini Kit (Qiagen, GmbH, Hilden, Germany). Animal handling was in accordance with national and international standards of animal welfare.
RNA concentrations were determined photometrically using Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription was performed on 1 μg of RNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, USA) and random hexamer primers (Promega, Madison, USA); RT mix was incubated for 60 min at 37°C. The relative abundance of different CaV subunit transcripts was assessed by TaqMan quantitative PCR (qRT-PCR) using a standard curve method based on PCR products of known concentration in combination with normalization using the most stable control genes as previously described [16]. TaqMan gene expression assays specific for all high-voltage activated Ca2+ channel subunits (α1, β, and α2δ) were designed to span exon–exon boundaries, were purchased from Applied Biosystems (Foster City, CA). The following assays were used [name (gene symbol), assay ID (Applied Biosystems)]: CaV1.1 (Cacna1s), Mm00489257_m1; CaV1.2 (Cacan1c), Mm00437953_m1; CaV1.3 (Cacna1d), Mm01209919_m1; CaV1.4 (Cacna1f), Mm00490443_m1; CaV2.1 (Cacna1a), Mm00432190_m1; CaV2.2 (Cacna1b), Mm00432226_m1; CaV 2.3 (Cacna1e), Mm00494444_m1; β1 (Cacnb1), Mm00518940_m1; β2 (Cacnb2), Mm00659092_m1; β3 (Cacnb3), Mm00432233_m1; β4 (Cacnb4) Mm00521623_m1; α2δ-1 (Cacna2d1), Mm00486607_m1; α2δ-2 (Cacna2d2), Mm00457825_m1; α2δ-3 (Cacna2d3), Mm00486613_m1; α2δ-4 (Cacna2d4), Mm01190105_m1. The endogenous control genes included were [name (gene symbol), assay ID (Applied Biosystems)]: γ-cytoplasmic actin (ACTB), Mm00607939_s1; beta-2-microglobulin (B2M), Mm 00437762_m1; glyceraldehyde-3-phosphate dehydrogenase (GAPD), Mm99999915_g1; hypoxanthine phosphoribosyl-transferase 1 (HPRT1), Mm00446968_m1; succinate dehydrogenase complex, subunit A (SDHA), Mm01352363_m1; tata box binding protein (TBP), Mm00446973_m1; transferrin receptor (TFRC), Mm00441941_m1. qRT-PCR (50 cycles) was performed in duplicates using 20 ng total RNA equivalents of cDNA and the specific TaqMan gene expression assay for each 20 μl reaction in TaqMan Universal PCR Master Mix (Applied Biosystems). Measurements were performed on at least three independent RNA preparations from each tissue and developmental stage. Analyses were performed using the 7500 Fast System (Applied Biosystems). The Ct values for each CaV gene expression assay were recorded for each individual preparation. To allow a direct comparison between the expression levels in different tissues, we normalized all experiments to Hprt1, Tfrc, and Sdha, which were determined to be most stably expressed reference genes across all preparations and time points [17]. Subsequently, normalized molecule numbers were calculated for each CaV subunit from their respective standard curve [16].
Data analysis and statistics
Data were organized and analyzed using MS Excel and SPSS (SPSS Inc., Chicago, IL, USA) or SigmaStat (Systat Software, Inc., San Jose, CA, USA) statistical software. Statistical significance was determined by a one-, two-, or three-way-ANOVA followed by Fisher or Holm-Sidak posthoc comparison, as indicated.
RESULTS
We have previously shown that the CaV1.2 α1-subunit is highly expressed in reactive astrocytes in a mouse model of AD [11]. Here we used tg mice overexpressing hAβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations to investigate whether this increased expression is related to the pathogenesis of Aβ plaque formation. To this end, we first characterized the onset and severity of Aβ plaque formation in wt and tg mice during aging. Second, we determined whether the altered Ca2+ channel expression is based on age- and Aβ -induced changes in the CaV1.2 α1-subunit expression pattern. Double and triple immunolabeling of wt and tg mice was employed to identify the specific morphological structure or cell type showing the increased Ca2+ channel expression. Finally, neurodegeneration and neuroinflammation induced by excitotoxic lesions served as a control to examine the role of astrogliosis on CaV1.2 α1-subunit expression independently of Aβ accumulation.
Immunohistochemistry for CaV1.2 α1-subunit, Aβ, GFAP, and Iba1
Tg mice harboring the London (V717I) and Swedish (K670M/N671L) mutations showed a strongly increased abundance of Aβ plaques at 11 months of age but not at early stages (2 and 4 months) when compared to wt control mice (Fig. 1C and D). Plaque formation was most evident in the frontal and parietal cortex but also affected the hippocampal formation and the amygdala (Table 1). Immunostaining of astroglial GFAP, microglial Iba1, as well as the L-type Ca2+ channel CaV1.2 α1-subunit, in 11 month-old mice identified remarkable differences between wt and tg animals (Fig. 1). In wt mice, CaV1.2 α1-subunit immunoreactivity could be detected in the cortex and the hippocampal formation, most pronounced in the molecular layer of the dentate gyrus and the CA3/CA2 regions, as previously described (Fig. 1A) [18, 19]. Similarly, Aβ immunostaining in wt mice revealed an evenly distributed pattern of immunoreactivity and, as expected, no aggregates, indicative of plaques, were found. The glial markers for astrocytes (GFAP, Fig. 1E) and activated microglia (Iba1, Fig. 1G) showed a scattered staining pattern throughout the hippocampal formation and the cortex. In 11 month-old tg animals, which displayed a strong formation of Aβ plaques, immunostaining for the CaV1.2 α1-subunit was strongly increased (Fig. 1B). At this stage the animals also displayed aggregated GFAP and Iba1 staining, indicative of the formation of reactive astrocytes and the accumulation of activated microglia, respectively (Fig. 1F and H).
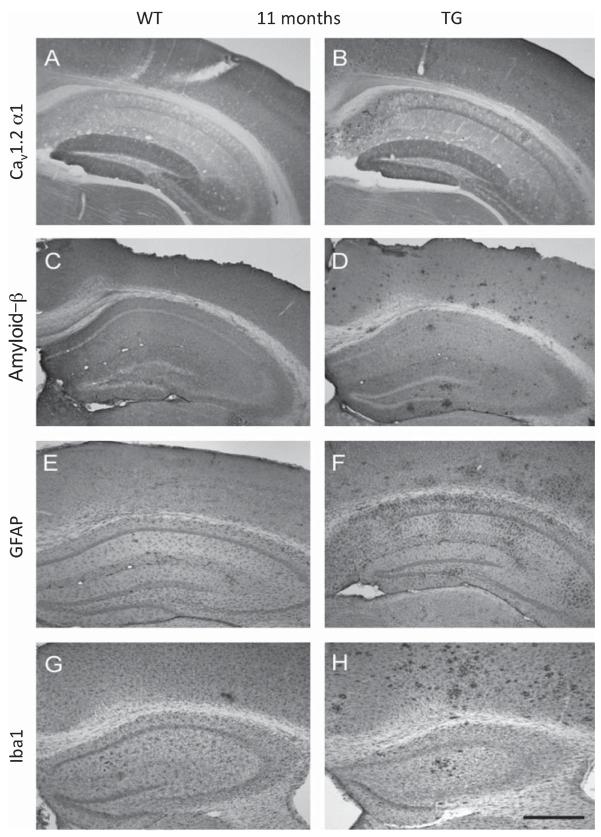
Different immunohistochemical staining patterns in 11 month-old wt and tg mice. Moderate CaV1.2 α1-subunit-like immunoreactivity (LI) in wt, especially in the dentate gyrus and CA2/CA3 regions (A) was observed, while an increased and cluster-like staining could be seen in tg mice, particularly in the cortex (B). Aβ-LI was markedly enhanced in the cortex of tg mice (D) whereas in wt almost no Aβ plaques were detectable (C). Astroglial GFAP-LI (E, F) and microglial Iba1-LI (G, H) revealed a scattered staining pattern throughout the whole cortex and hippocampus in wt (E and G) compared to a cluster-like distribution in tg mice (F and H). Scale bar in H represents = 700 μm.
Table 1
Number of amyloid-β plaques in transgenic (tg) animals compared to wild type (wt) controls
| Area | Age (months) | wt plaques/mm2 | tg plaques/mm2 |
|---|---|---|---|
| Hippocampus | 2 | 0.2 ± 0.2 | 0.7 ± 0.4 |
| 4 | 0.0 ± 0.0 | 1.3 ± 0.7 | |
| 11 | 0.4 ± 0.4 | 11.3 ± 4.5*** | |
| Amygdala | 2 | 0.7 ± 0.3 | 0.2 ± 0.1 |
| 4 | 0.1 ± 0.1 | 0.5 ± 0.3 | |
| 11 | 1.0 ± 0.3 | 5.3 ± 1.6*** | |
| Parietal Cortex | 2 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| 4 | 0.0 ± 0.0 | 0.3 ± 0.2 | |
| 11 | 0.3 ± 0.1 | 17.7 ± 1.5*** | |
| Frontal Cortex | 2 | 0.3 ± 0.2 | 0.2 ± 0.1 |
| 4 | 0.5 ± 0.3 | 0.2 ± 0.2 | |
| 11 | 0.7 ± 0.4 | 20.4 ± 5.2*** |
Eleven month-old tg mice showed a highly significant increase of amyloid-β plaques measured in four different brain areas. Four animals per group and time point were analyzed. Statistical analysis was performed by one way ANOVA with a subsequent Fisher PLSD posthoc test. Data represent mean±SEM.
RT-PCR mRNA expression profile of LTCC subunits
The strong increase of CaV1.2 α1-subunit immunoreactivity in 11 month-old brains of tg mice suggests an involvement of Ca2+ channels in the process of Aβ plaque formation. To test whether this apparent upregulation involves changes in gene expression of CaV1.2 α1-subunits or any other Ca2+ channel subunits, we analyzed the mRNA expression profiles of all high voltage-activated Ca2+ channel α1, β, and α2δ subunits in mouse hippocampus from 2, 4, and 11 month-old wt and tg mice (Fig. 2), as previously described [16]. Wt and tg hippocampi expressed all CaV α1-subunits except CaV1.1 and CaV1.4 as well as all auxiliary Ca2+ channel subunits except α2δ-4. Maturation and aging was accompanied by a significant increase in the total number of Ca2+ channel subunit transcripts (Fig. 2) as well as a specific developmental increase of CaV2.1 and CaV2.2 between 2 and 4 months. Nevertheless, between wt and tg mice, we neither observed an overall difference in the total Ca2+ channel expression levels, nor a difference within each age group, nor in any specific Ca2+ channel subunit. This indicates that the increased CaV1.2 protein expression in Aβ plaque forming tg animals is not based on general or specific effects of the hAβPP mutations acting at the level of Ca2+ channel gene expression. In contrast, these results extend the previously observed stability of Ca2+ channel expression to aging mice [16]. Together these results indicate that apparent changes in Ca2+ channel expression and localization correspond to the onset of Aβ plaque formation and thus may occur at the cellular and posttranscriptional level.
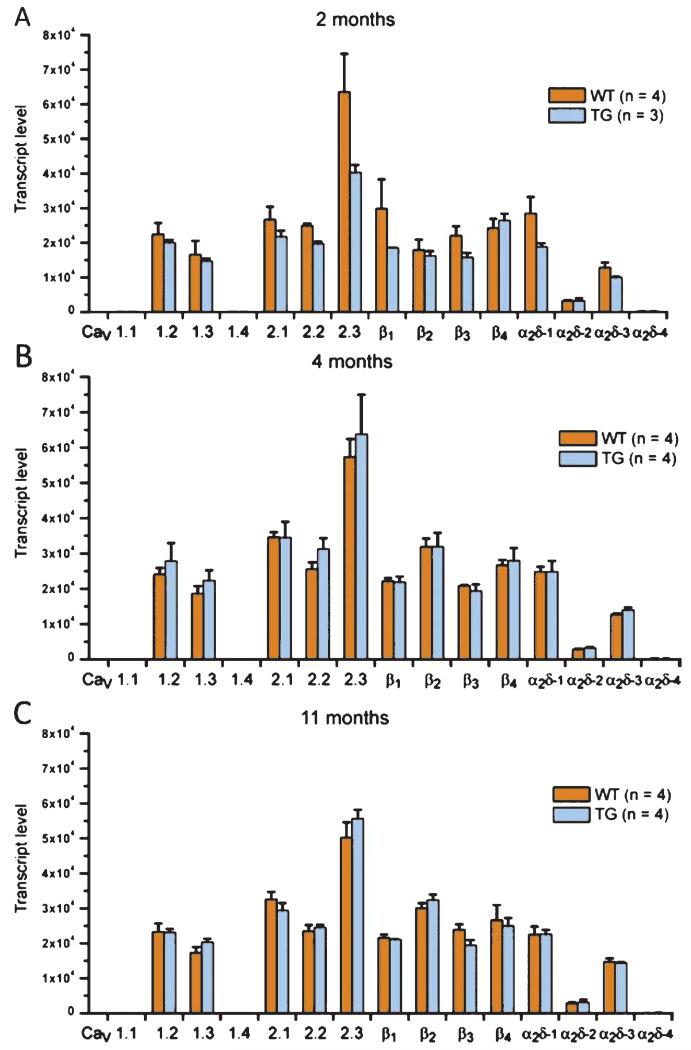
Real time PCR analysis reveals no differences in the L-type Ca2+ channel (LTCC) subunit mRNA expression profiles between wt and tg mice. Expression profiles of the high voltage-activated Ca2+ channel α1, β, and α2δ subunits in mouse hippocampus from 2 (A), 4 (B), and 11 (C) month-old wt (orange) and tg (blue) mice. All CaV α1 subunits except CaV1.1 and CaV1.4 as well as all auxiliary calcium channel subunits except α2δ-4 were reliably expressed. Total Ca2+ channel subunit expression was significantly higher in 4 month-old mice compared to 2 month and 11 month (p < 0.001). Both CaV2.1 and CaV2.2 mRNAs showed a specific developmental increase from 2 months to 4 months (p < 0.001 for CaV2.1 and p = 0.014 for CaV2.2). However, between wt and tg mice, there was neither an overall difference in the total expression levels nor a difference within an age group or between a specific Ca2+ channel subunit mRNA. [3-way ANOVA: age, F(2) = 25.1, p < 0.001; gene, F(11) = 257.2, p < 0.001; genotype, F(1) = 0.5, p = 0.47; age*gene, F(22) = 1.6, p < 0.05; age*genotype, F(2) = 2.4, p = 0.09; age*gene*genotype, F(22) = 0.5, p = 0.95; were applicable (see above) p-values are derived from Holm-Sidak posthoc analysis; data are presented as mean; error bars: ± SEM.]
CaV1.2 α1-subunit expression in astrocytes around Aβ plaques
Since the observed changes in the Ca2+ channel staining pattern which associated with Aβ plaque formation were independent of the overall gene expression level, we next sought to characterize the changes in relation to specific and well characterized markers in AD pathology. Immunofluorescence labeling in 11 month-old wt mice revealed CaV1.2 α1-subunit immunopositive cells scattered throughout the entire cortex (Fig. 3A). As expected from their predominant neuronal role, CaV1.2 α1-subunit staining was not specifically associated with GFAP staining (Fig. 3A, C and E). In the cortex of 11 month-old tg mice, however, the distribution pattern of CaV1.2 α1-subunit immunoreactivity (Fig. 3B) was more similar to that of GFAP staining (Fig. 3D), which is typically associated with Aβ plaques. Interestingly, a considerable proportion of CaV1.2 staining colocalized with GFAP-positive cells, which showed the morphological phenotype typical for reactive astrocytes (Fig. 3B, D and F; Fig. 5). Furthermore, in 2 and 4 month-old tg mice, the number of CaV1.2 α1-subunit immunopositive cells was not different from wt animals (data not shown, n = 4 per group).
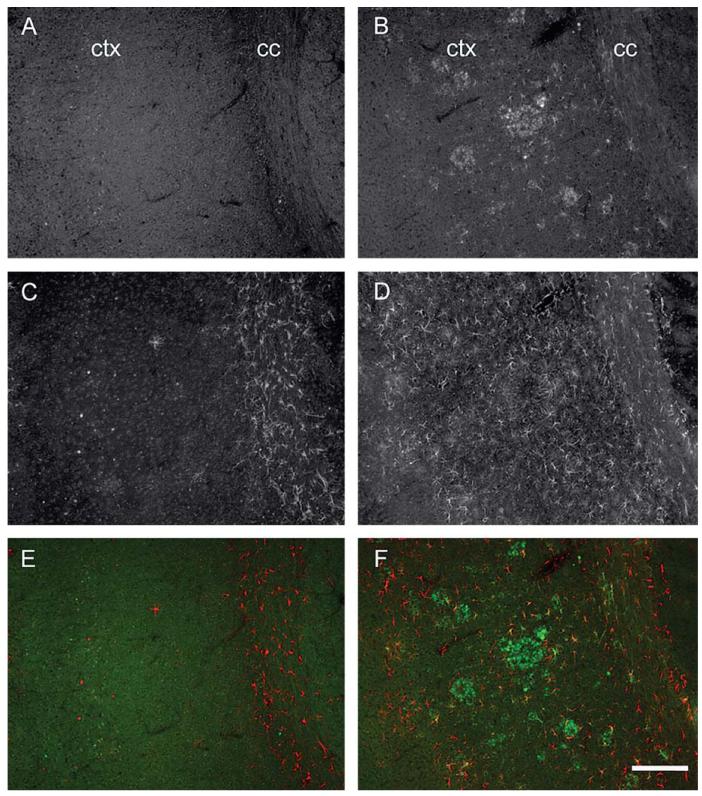
Increased CaV1.2 α1-subunit and astroglial GFAP expression in the cortex of 11 month-old tg mice. In the cortex and corpus callosum of 11 month-old wt mice, CaV1.2 α1-like immunoreactivity (-LI) was homogenously distributed (A) whereas in tg animals of same age, a cluster-like CaV1.2 staining pattern was evident (B). In wt brain sections, GFAP-positive astrocytes were primarily located in the corpus callosum (C), while tg animals displayed severely enhanced GFAP-LI spread throughout the entire cortex and corpus callosum (D). Merged images for wt (E) and tg (F) mice (CaV1.2-LI, green; GFAP-LI, red). ctx, cortex; cc, corpus callosum. Scale bar in F represents = 180 μm.
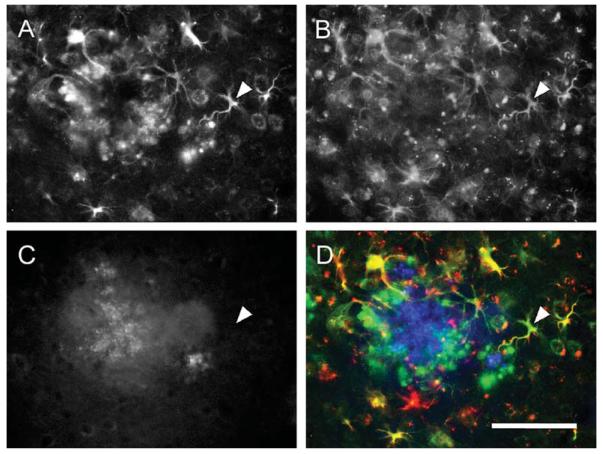
Triple fluorescent immunohistochemistry reveals the association of CaV1.2 α1-subunit-positive astrocytes with amyloid-β (Aβ) plaques in aging tg mice. The increased CaV1.2-immunoreactivity in the cortex of an 11 month-old tg mouse (A) colocalizes in a large part with GFAP-positive astrocytes (indicated by a white arrowhead, B), surrounding an Aβ plaque (C). Merged image (D) showing CaV1.2 (green), GFAP (red), and Aβ (blue). Scale bar in D represents = 100 μm.
Immunohistochemistry for the β4 subunit
The plasma membrane expression of CaV1.2 Ca2+ channels is regulated by the amount of available auxiliary β subunits [19, 20]. Because the auxiliary β4 subunit isoform is strongly expressed in the cortex [16, 21] and is a possible candidate for an interaction with CaV1.2 channels, we analyzed the distribution of β4 immunoreactivity in the cerebellum of an 11 month-old wt mouse. As expected, β4 immunoreactivity was most evident in the molecular layer of the cerebellum (Fig. 4A). As a control, the cerebellum of the β4 mutant lethargic mouse was stained for β4 and showed markedly reduced immunoreactions, particularly in the molecular layer (Fig. 4B). In the hippocampus of wt and tg animals, the most prominent β4 staining was observed within the pyramidal cell layer (Fig. 4C). In CA3, CA2, CA1, and the dentate gyrus, intensely stained isolated β4-immunoreactive cells were evident (Fig. 4D). These cells did not colocalize with GFAP and according to their size and morphology represent most likely neurons (Fig. 4E, F, and G). In contrast to CaV1.2, 11 month-old tg mice did not show a higher expression level of β4 than wt animals, and accordingly no association of β4 with GFAP aggregates was found near Aβ plaques.
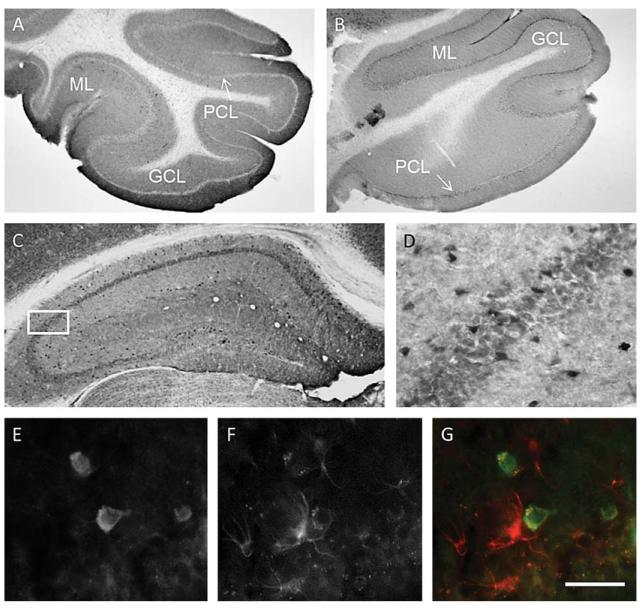
Immunoreactivity of the auxiliary Ca2+ channel β4 subunit does not co-localize with reactive astrocytes. The immunohistochemical staining for β4 in the cerebellum of an 11 month-old wt mouse showed an intense immunoreactivity most evident in the molecular layer (A). As expected, immunohistochemistry revealed a markedly reduced staining intensity in the cerebellar molecular layer of a 2 week-old β4 mutant lethargic mouse (lh) (B). In both, 11 month-old wt and tg mice, isolated neurons with strong β4 immunostaining were detected in hippocampal CA1, CA2, and CA3 areas (C and in the magnified selection in E, taken from a wt mouse). These β4-positive cells (D and E) did not colocalize with GFAP-positive astrocytes (F and G). GCL, granular cell layer; ML, molecular layer; PCL, pyramidal cell layer. Scale bar in G represents = 500 μm (A, B); 300 μm (C); 130 μm (D) and 50 μm (E, F, G).
CaV1.2 α1-subunit expression in relation to Aβ positive plaques
The staining pattern of CaV1.2 α1-subunits in relation to GFAP-positive cellular aggregates suggested a preferential association with Aβ immunopositive plaques. Indeed a triple labeling immunofluorescence experiment unambiguously demonstrates the high expression of CaV1.2 α1-subunits in reactive astrocytes (GFAP-positive) surrounding an Aβ plaque (Fig. 5). Quantification shows that in 11 month-old tg mice, about 90% of Aβ immunopositive structures were associated with CaV1.2 α1-subunit immunoreactivity. In less than 5% Aβ-positive structures were not associated with CaV1.2 α1-subunits (Table 2).
Table 2
Association of Aβ plaques with the CaV1.2 α1-subunit
| Percentage [%] | |
|---|---|
| Aβ+ CaV 1.2− | 3.5 ± 1.7 |
| Aβ+ CaV 1.2+ | 88.8 ± 1.0 |
| Aβ− CaV1.2+ | 7.7 ± 1.3 |
Double immunohistochemistry revealed a strong association of Aβ plaques with aggregates of CaV1.2 α1-subunits. Both, Aβ plaques and CaV1.2 α1-subunit-positive structures were counted under a 20× magnification. Four brains (tg, 11 months) were analyzed and on average 8 microscopic fields were randomly selected within the cortex (each field had an area of 1.1 mm2). Per field on average 10 plaques were counted, giving a total of approximately 80 analyzed plaques per brain. Statistical analysis was performed by one way ANOVA with a subsequent Fisher PLSD posthoc test. Data represent mean±SEM.
Astroglial CaV1.2 α1-subunit expression after quinolinic acid lesion
The expression pattern of CaV1.2 α1 subunits in relation to reactive GFAP-positive astrocytes and neuronal cells around Aβ plaques in tg mice suggests an involvement of CaV1.2 channels in neurodegeneration associated with AD pathology. The increased CaV1.2 α1-subunit expression in astrocytes surrounding the plaques could be a specific feature of reactive astrocytes or may be secondary to the Aβ deposition. Thus, in order to test a possible recruitment of CaV1.2 channels in reactive astrocytes in a model of neurodegeneration and neuroinflammation independent of Aβ, we induced excitotoxic lesions in 6 month-old mice by stereotactic injections of QA into the striatum. Three brains each with 3–5 sections per brain were analyzed. In QA-lesioned animals, a prominent increase in the number and density of GFAP expressing cells, indicating astrogliosis, occurred at the site of QA injection (ipsilateral; Fig. 6A and C), whereas in the opposite hemisphere without injection (contralateral, Fig. 6B), no increase of GFAP-positive cells could be detected. Most importantly, this increase of GFAP expressing cells was not accompanied by an increase of CaV1.2 immunoreactive cells at the site of lesion (Fig. 6E-G), indicating the specificity of CaV1.2 expression in reactive astrocytes associated with plaques.
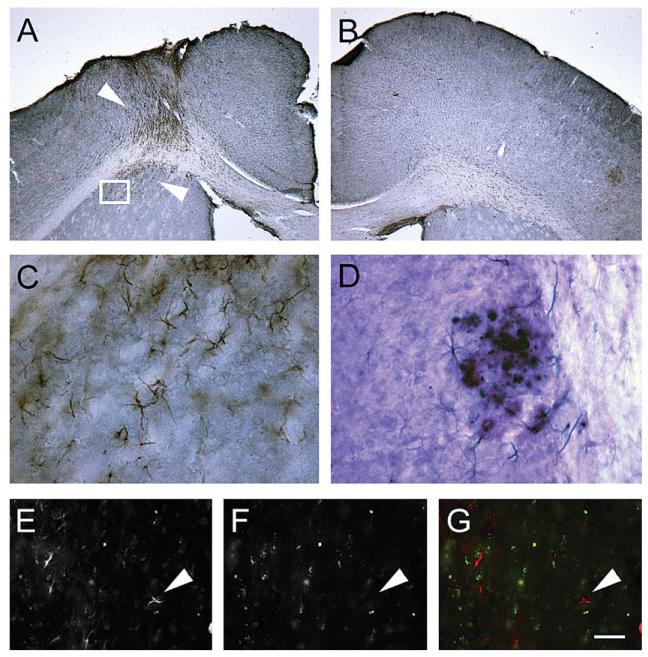
Quinolinic acid (QA) induced lesions showed no upregulation of CaV1.2 α1-subunits in GFAP-positive reactive astrocytes in 6 month-old wt mice. Upregulation of GFAP-like immunoreactivity (LI) at the ipsilateral lesion site is shown in A (layer of brown cells indicated by arrowheads). As a control, the controlateral site of the same mouse brain without injection displayed no upregulation of GFAP-positive astrocytes (B). Double-labeling indicates that CaV1.2 is not upregulated in GFAP immunopositive cells (C; taken from an aspect indicated in A), whereas, CaV1.2 α1-subunit immunostaining is intense around amyloid-β plaques (picture taken from an 11 month-old tg mouse). Fluorescent immunohistochemistry also shows that CaV1.2 (E) does not colocalize with lesion-induced reactive astrocytes (GFAP, F, arrowheads in the merged image, G). Note: In a picture taken from a tg mouse, a colocalization of CaV1.2 (blue cells) in astrocytes around Aβ (violet plaque) in 11 month-old tg mice as revealed in a double immunochromogenic staining (D; see also Fig. 5). Scale bar in G represents = 700 μm (A, B); 200 μm (C, D) and 100 μm (E-G).
DISCUSSION
In the present study we investigated the expression of CaV1.2 α1-subunits in brains of AD model mice. We observed that the high expression of CaV1.2 α1-subunits in reactive astrocytes depended on the presence of Aβ plaques, as there was no expression prior to plaque formation or in inflammation-induced reactive astrocytes.
Increased expression of astroglial CaV1.2 in reactive astrocytes around plaques
One of the major hallmarks in AD are Aβ plaques in the brain. Several mouse models are useful to study the expression and localization of the plaques in the brain [22], including our well established model expressing the hAβPP with the London and Swedish mutations [11]. In the present study, we extend our previous findings and show a detailed time and brain region specific development of the plaques. We found that the majority of the plaques develop in cortex and hippocampus only after 11 months. Thus, in the present study we focused mainly on CaV1.2 α1-subunit expression at this late time point in these both brain areas. As already shown [10], we strengthen our findings that CaV1.2 α1-subunit immunoreactive cells are markedly enhanced in the vicinity of plaques (>90%) in 11 month-old animals. Based on the cell morphology and co-staining with GFAP, these cells have been proposed to be reactive astrocytes [23]. Further, we provide evidence for this cell identity by excluding the possibility that these CaV1.2 α1-subunit/GFAP-positive cells were microglia, because these cells did not stain for the microglial marker Iba1 [24].
This leads to the question whether the astroglial CaV1.2 α1-subunit expression is caused by the AD pathology (including inflammation, oxidative stress, or Ca2+ dysregulation) [25]; or if it is caused directly by the plaque generation caused by the Aβ cascade [26]. In the latter case, the question arises whether the plaques per se (containing aggregated Aβ peptides) or the soluble Aβ peptides alone may induce the astroglial CaV1.2 α1-subunit expression. And third, it is possible that the CaV1.2 α1-subunit expression is a consequence of the activation and reaction of the astrocytes [27] independent of plaque formation.
Aberrant Ca2+ homeostasis caused by increased CaV1.2 channel expression in the AD brains [28] could be possibly related to the generation of plaques. In this case we would have expected to detect CaV1.2 α1-subunit-positive cells already in the pre-plaque stage of 2 and 4 month-old animals. However, our data clearly show that no astroglial CaV1.2 α1-subunit-positive cells were detectable in astrocytes at this stage. In addition, based on our experiments with quinolinic acid we also exclude that the CaV1.2 α1-subunit expression is just a simple upregulation in reactive astroglia in the brain. This is partly in contrast to a study, reporting that rather unspecific brain injury, hypomyelination or ischemia upregulates LTCC channels in reactive astrocytes [29]. Thus, we favor the idea that indeed the plaque is responsible for the activation of reactive astroglia and the subsequent upregulation of the CaV1.2 α1-subunits. We further suggest that the induction of GFAP-positive cells and the upregulation of CaV1.2 α1-subunits in these cells are two independent mechanisms and that the latter is specifically caused by the Aβ burden in AD tg mouse brains. Further, we conclude that the increased expression of CaV1.2 α1-subunits in reactive astrocytes is part of the AD phenotype in the tg mouse model.
Astroglial CaV1.2 is not accompanied with Ca2+ channel gene expression
The conclusion that increased Ca2+ channel expression is not a cause but an effect of Aβ plaques is further supported by our quantitative RT-PCR analysis of Ca2+ channel transcripts. If mutated AβPP would cause increased CaV1.2 α1-subunit expression [30], which in turn contributed to the generation of the AD phenotype, increased CaV1.2 mRNA levels should have preceded the appearance of CaV1.2 α1-subunit immunoreactive cells. However, CaV1.2 mRNA was neither increased in tg brains at the pre-plaque stage nor in mouse brains that had already developed the AD phenotype. Interestingly, none of the other examined subunit isoforms of high voltage activated Ca2+ channels was different in tg brains compared to matched wt brains. Thus, the mutations in AβPP leading to AD pathology in mice have no impact on the expression of any of the Ca2+ channels, nor did the increased expression of CaV1.2 α1-subunits observed in immunocytochemistry cause compensatory down-regulation of other Ca2+ channel genes. Together these findings disagree with a causal involvement of CaV1.2 or other high voltage activated Ca2+ channels in the pathogenesis of AD as put forth in the Ca2+ hypothesis of neurodegenerative disease [1].
On the contrary, the increased Aβ load in the plaques may cause the activation of astrocytes in the vicinity of the plaque and/or the upregulation of CaV1.2 α1-subunits in these cells. This upregulation could occur at the protein level by increased translation or decreased degradation of the channel. Alternatively, if CaV1.2 α1-subunit transcriptional regulation in astrocytes was involved, it may have escaped detection by qRT-PCR, because of the relatively small percentage of CaV1.2 α1-subunit expression by reactive astrocytes in the background of overall high Ca2+ channel expression levels in neurons. Therefore, in order to test the specificity of the increased CaV1.2 α1-subunit expression we also performed immunohistochemistry for the auxiliary β4 subunit. The specificity of the β4 subunit staining was confirmed in cerebellum and hippocampus of wt and knockout mice as positive and negative controls, respectively. Although the examined β4 subunit is only one of four possible partners in the channel complex [29-32], the absence of this β isoform in the reactive astrocytes indicates that the increased CaV1.2 α1-subunit levels are not the result of a general overexpression of Ca2+ channels. Nevertheless, a possible involvement of other auxiliary calcium channel subunits (β and α2δ) needs to be addressed in future studies. To this end, it will be necessary to ultimately evaluate the suitability of available antibodies for immunohistochemistry by establishing respective knockout control tissues, similar to the β4 subunit antibody used in this study.
Function of CaV1.2 α1-subunit in reactive astrocytes and its possible role in AD
In recent years glia and specifically astrocytes have been ascribed an increasingly active role in brain function [23, 27, 33, 34]. One reason is the realization that astrocytes express many ion channels including voltage activated Ca2+ channels [35-38] and that they regulate trophic functions of neurons as well as neuronal network activity [39, 34]. Whereas our present findings do not support a role of CaV1.2 α1-subunits in early stages of AD, it will be important to determine whether and how they influence Ca2+ homeostasis in the diseased brain and whether blocking them may counteract the aggravation of neurodegeneration and dementia. Further experiments to clarify the role of CaV1.2 channels in reactive astrocytes are necessary. In order to address the subunit composition of these channels, in situ hybridization may provide further evidence on the CaV1.2 α1-subunit expression around plaques. Second, in order to investigate whether soluble or aggregated Aβ is responsible for the activation of CaV1.2 α1-subunits, experiments in primary isolated astrocytes will be necessary, including models to selectively induce reactive astroglia. And finally, electrophysiological experiments, e.g., in organotypic brain slices containing plaques, may prove the physiological function of the astroglial CaV1.2 channels in the AD brain.
It needs to be pointed out that the presence of CaV1.2 α1-subunits does not imply the presence of functional LTCC-mediated ion conductances in these cells. Such a role would have been strengthened by the presence of a β-subunit, such as β4, which was not the case. However, CaV1.2 α1-subunits can be processed C-terminally and a C-terminal fragment can modulate transcription [40]. Thus, we cannot exclude that reactive astrocytes may likely use such a signaling pathway without any accessory subunit. However, so far in the absence of electrophysiological data the question of a functional CaV1.2 channel still remains open. Further experiments are under way to label astrocytes in vivo to (1) microdissect reactive astrocytes for single cell RT-PCR for auxiliary subunits and (2) to perform electrophysiology in brain slices of reactive astrocytes.
CONCLUSION
In conclusion, CaV1.2 α1-subunits are strongly expressed in reactive astrocytes in 11 month-old tg mice overexpressing human AβPP with London and Swedish mutations. This high amount of CaV1.2 α1-subunits in astrocytes is likely to be a consequence of the increasing Aβ burden. A critical question to be addressed in future is whether the higher availability of CaV1.2 α1-subunits in reactive astrocytes is functionally active and if CaV1.2 channels in reactive astrocytes could be a target for a neuroprotective strategy [41, 42].
ACKNOWLEDGMENTS
We are thankful to Ursula Kirzenberger-Winkler and Roman Egger for technical assistance. This study was supported by the Sonderforschungsbereich SFB F4405-B19 and F4404-B19 as well as P24079-B21 of the Austrian Science Fund.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1810).
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.3233/jad-130560
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4312770?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Dysregulation of Ion Channels and Transporters and Blood-Brain Barrier Dysfunction in Alzheimer's Disease and Vascular Dementia.
Aging Dis, 15(4):1748-1770, 01 Aug 2024
Cited by: 3 articles | PMID: 38300642 | PMCID: PMC11272208
Review Free full text in Europe PMC
Identification of methylation-regulated genes modulating microglial phagocytosis in hyperhomocysteinemia-exacerbated Alzheimer's disease.
Alzheimers Res Ther, 15(1):164, 03 Oct 2023
Cited by: 0 articles | PMID: 37789414 | PMCID: PMC10546779
Modulation of L-type calcium channels in Alzheimer's disease: A potential therapeutic target.
Comput Struct Biotechnol J, 21:11-20, 26 Nov 2022
Cited by: 7 articles | PMID: 36514335 | PMCID: PMC9719069
Review Free full text in Europe PMC
Microglial ion channels: Key players in non-cell autonomous neurodegeneration.
Neurobiol Dis, 174:105861, 14 Sep 2022
Cited by: 7 articles | PMID: 36115552 | PMCID: PMC9617777
Review Free full text in Europe PMC
Enhanced long-term potentiation and impaired learning in mice lacking alternative exon 33 of CaV1.2 calcium channel.
Transl Psychiatry, 12(1):1, 10 Jan 2022
Cited by: 7 articles | PMID: 35013113 | PMCID: PMC8748671
Go to all (26) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
L-type calcium channel CaV 1.2 in transgenic mice overexpressing human AbetaPP751 with the London (V717I) and Swedish (K670M/N671L) mutations.
J Alzheimers Dis, 20(4):1167-1180, 01 Jan 2010
Cited by: 19 articles | PMID: 20413896
L-type calcium channel blockers and substance P induce angiogenesis of cortical vessels associated with beta-amyloid plaques in an Alzheimer mouse model.
Neurobiol Aging, 36(3):1333-1341, 31 Dec 2014
Cited by: 22 articles | PMID: 25619662 | PMCID: PMC4347662
Early Activation of Astrocytes does not Affect Amyloid Plaque Load in an Animal Model of Alzheimer's Disease.
Neurosci Bull, 34(6):912-920, 21 Jul 2018
Cited by: 7 articles | PMID: 30032411 | PMCID: PMC6246842
Fatty acids as biomodulators of Piezo1 mediated glial mechanosensitivity in Alzheimer's disease.
Life Sci, 297:120470, 10 Mar 2022
Cited by: 8 articles | PMID: 35283177
Review
Funding
Funders who supported this work.
Austrian Science Fund FWF (4)
Synapses and disease in Ca2+ channel alpha2delta mouse models
Privatdozent Dr. Gerald OBERMAIR, Medical University of Innsbruck
Grant ID: P 24079
L-type Ca2+ channels in reactive astroglia in CNS disease
Univ.Prof. Dr. Christian HUMPEL, Medical University of Innsbruck
Grant ID: F 4405
Cav1.3 signaling complexes and epigenetic mechanisms
Univ.Prof. Dr. Bernhard E. FLUCHER, Medical University of Innsbruck
Grant ID: F 4406
Alpha-Synuclein and ligodendroglia in MSA pathogenesis
Prof. Dr. Nadia STEFANOVA, Medical University of Innsbruck
Grant ID: P 25161

