Abstract
Free full text

Combined juvenile polyposis and hereditary hemorrhagic telangiectasia
Juvenile polyposis (JP or JPS for juvenile polyposis syndrome) is an autosomal dominant disorder that often presents in childhood. It is characterized by the presence of hamartomatous (juvenile) polyps that vary in number from five to several hundred (1, 2). The polyps are found primarily in the colorectum, but they can be present throughout the gastrointestinal tract, from the stomach to the rectum (2). Even though these polyps are normally benign, patients have an increased risk of gastrointestinal cancer (1–3). This disease occurs in approximately 1 in 100,000 people (4), and in 50% to 60% of the patients a germline mutation in SMAD4 (5) or bone morphogenic protein receptor 1A (BMPR1A) (6) genes can be found.
Hereditary hemorrhagic tel-angiectasia (HHT; Osler-Weber-Rendu syndrome) is another autosomal dominant disorder distinguished by vascular dysplasia in multiple organs that can result in excessive bleeding. This syndrome was initially described by Osler in 1901 in a report of a familial form of recurrent mucous membrane bleeding from telangiectasias (3). Characteristic features include telangiectasias of the skin and oral and nasal mucosa, epistaxis, and arteriovenous malformations (AVMs) of the lungs, liver, brain, and gastrointestinal tract that can lead to hemorrhage and stroke (7–9). The frequency of this syndrome differs between populations, but it ranges from 1 in every 1300 Afro-Caribbeans in the Netherlands Antilles (10) to 1 in every 40,000 people in northern England (4). Approximately 80% of the families have a mutation in the endoglin (ENG) (5) or activin receptor-like kinase 1 (ALK1) (6) genes, while the remaining 20% of patients have a mutation in the SMAD4 gene (7) or in new loci mapped to chromosome 5 and chromosome 7 (8).
A syndrome that combines JP and HHT was first described in 1980 (9, 10). These patients exhibit symptoms of both syndromes to varying degrees. It was not until 2004 that this combined syndrome was found to be associated with a mutation in the SMAD4 gene (7). In addition to the previously mentioned findings, these patients are more likely to develop early onset colorectal cancer (CRC; mean age 28 years) and have a higher rate of anemia than HHT patients with other mutations (11). Herein we describe a patient with this combined syndrome, JP and HHT, who presented to our emergency department.
CASE REPORT
A 27-year-old woman presented to the emergency room at Baylor University Medical Center at Dallas with complaints of diarrhea, fatigue, hematochezia, nausea, vomiting, and abdominal pain. She had been experiencing these symptoms on and off for years with a progressive worsening of her symptoms. Her past medical history was significant for intermittent small bowel obstructions and gastrointestinal bleeding requiring a laparotomy with intraoperative enteroscopy and resection of three “inflammatory polyps.” She also had a history of epistaxis and chronic iron deficiency anemia with low levels of albumin requiring frequent blood transfusions. She complained that she had been “sick all of her life.” Early in 2011, she had been treated with steroids, azathioprine, and infliximab for Crohn's disease, but she showed no improvement in symptoms. Her family history included a father with a “large AVM in his head” and nosebleeds and a paternal grandfather who had “AVMs” and died of a “heart attack” in his mid 50s (Figure (Figure11).
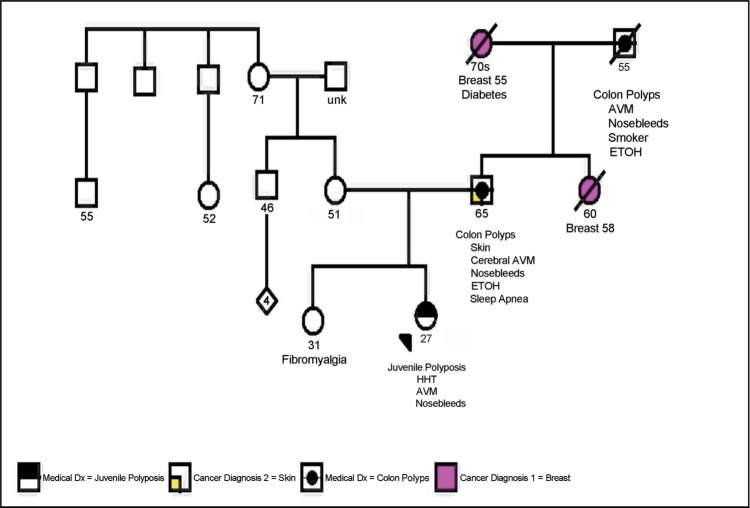
Patient's pedigree. Proband's father and paternal grandfather both had colon polyps, arteriovenous malformations (AVMs), and nosebleeds, while her maternal lineage was unremarkable for juvenile polyposis syndrome or hereditary hemorrhagic telangiectasia (HHT). These results suggest that the patient's putative SMAD4 mutation was paternally inherited.
The patient appeared chronically ill and had Cushingoid features. She had clubbing of the fingers and toes (Figure (Figure22), an abdominal bruit, diffuse abdominal tenderness to palpation, and one single, small telangiectasia on her lip. Bone marrow biopsy indicated the patient had an iron deficiency anemia. Though a more remote computed tomography (CT) scan showed terminal ileal thickening suggestive of Crohn's disease, a CT enterography revealed numerous nodular polypoid arterial enhancing lesions of the distal and terminal ileum, a markedly enlarged hepatic artery as can be seen with HHT, and small subcapsular foci of liver enhancement suggestive of hepatic AVMs (Figure (Figure33), which were later shown to be AVMs on hepatic magnetic resonance imaging. The patient underwent upper endoscopy that was normal and a colonoscopy that showed a few inflammatory polyps in the colon and multiple inflammatory polyps in the terminal ileum. There was a large ileal polyp that intermittently prolapsed through the ileocecal valve (Figure (Figure4a4a). Double balloon colonoscopy with retrograde enteroscopy showed multiple large polypoid lesions in the ileum forming a mass-like lesion (Figures (Figures4b4b and and4c4c). It was determined that due to the large polyp burden and mass-like effect, endoscopic resection of the polyps would not be possible. It was thought that the patient was likely experiencing intermittent bowel obstruction and bleeding from these lesions and was therefore referred for surgical resection of the polypoid mass.
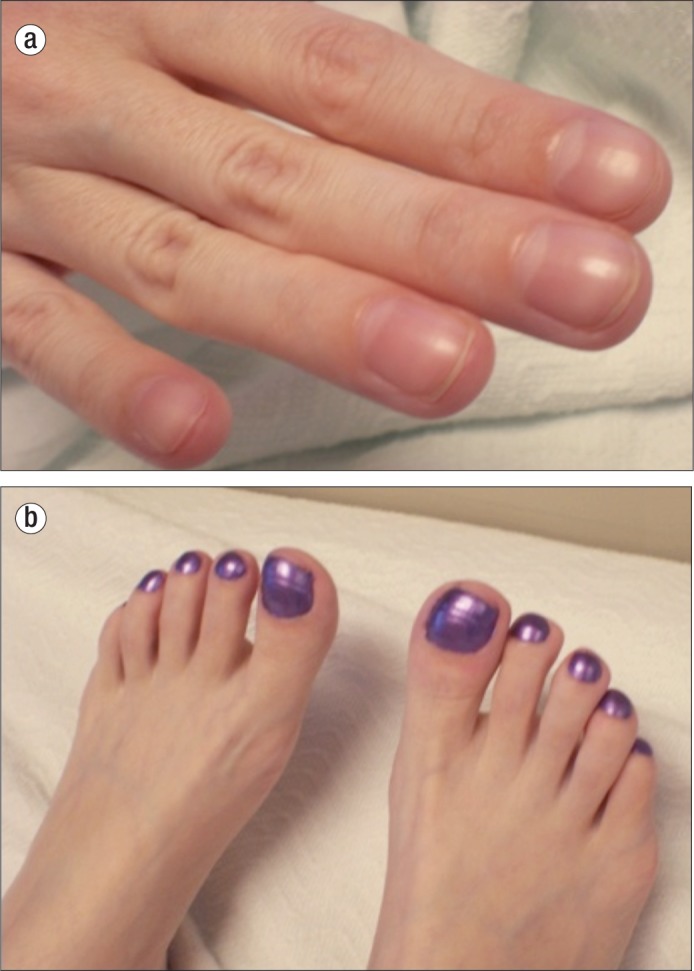
Clubbing of the (a) fingernails and (b) toes in a patient with putative juvenile polyposis–hereditary hemorrhagic telangiectasia syndrome. Both traits are typical of hereditary hemorrhagic telangiectasia.

Computed tomography images of the patient with juvenile polyposis–hereditary hemorrhagic telangiectasia (HHT) syndrome. Both (a) an enlarged hepatic artery (arrow) and (b) the portal venous enhancement pattern of the liver (arrows) are consistent with arteriovenous malformations normally seen in the liver of patients with HHT. Observe in (c) multiple enhancing small bowel polyps in a segment of distal ileum.

Results of colonoscopy: (a) a large juvenile polyp prolapsing through the ileocecal valve into the cecum (arrow); (b, c) multiple polypoid lesions forming a mass in the terminal ileum (arrows). These results are consistent with a diagnosis of juvenile polyposis.
Small bowel and colon biopsies showed the presence of hamartomatous/juvenile polyps. The fragments of tissue showed surface ulceration and an underlying inflammatory infiltrate (Figure (Figure5a5a). The deeper portions of the glands demonstrated crowded and hyperchromatic nuclei (first thought to be adenomatous change), but there was relatively normal maturation towards the surface, suggesting a reactive process. These were thought to most likely represent hamartomatous polyps with regenerative changes.
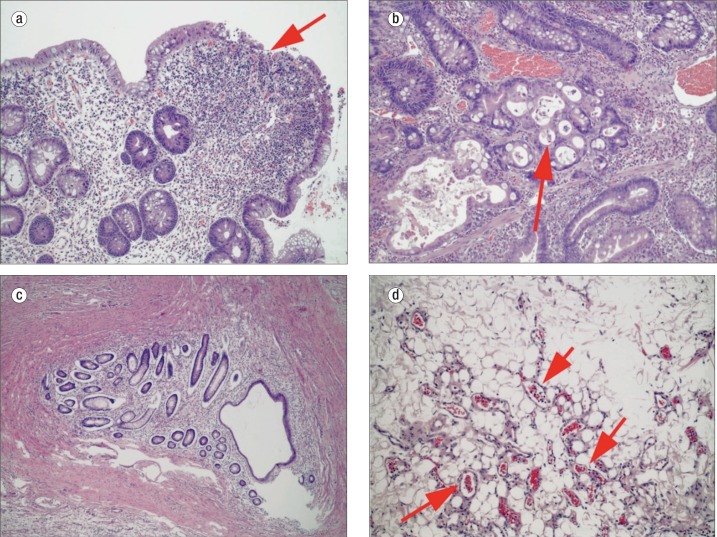
Histology illustrating traits of juvenile polyposis syndrome (JPS) and hereditary hemorrhagic telangiectasia (HHT) in the same patient: (a) hamartomatous polyp with surface ulceration (arrow), inflammation, and reactive changes, consistent with JPS; (b) polyp with focus concerning for invasive carcinoma (arrow), consistent with JPS; (c) enteritis cystica profunda, suggesting repeated cycles of inflammation and regeneration, again consistent with JPS; and (d) mesenteric adipose tissue with a proliferation of small dilated blood vessels (arrows), consistent with HHT.
The patient then underwent right hemicolectomy with resection of the affected portion of her small bowel. Approximately 2 feet of terminal ileum and right colon were resected. Histologic sections showed similar findings to that seen on biopsy, with hamartomatous polyps containing regenerative changes. Some areas contained more worrisome architectural features with complex cribriforming of glands (high-grade dysplasia) and focal gland breakdown, suspicious for early invasion (Figure (Figure5b5b). Rare foci showed more definitive evidence of invasion with small clusters and single cells extending into the lamina propria. No invasion was seen beyond the lamina propria, consistent with an early “intramucosal adenocarcinoma.” Additionally, there were 22 benign lymph nodes, along with the incidental finding of enteritis cystica profunda (Figure (Figure5c5c). Multiple hemangiomas and vascular changes (Figure (Figure5d5d), consistent with the clinical history of HHT, were also present. The patient did well following surgery and has been referred to a genetic counselor for testing and follow-up.
DISCUSSION
JP-HHT syndrome is a combination of two autosomal dominant disorders secondary to a mutation in one gene, SMAD4. Both syndromes alone are rare, with an incidence of 1 in 100,000 for JPS (12) and 1 in 1300 to 1 in 40,000 for HHT based on ethnicity (4, 13). Approximately 50% to 60% of patients with JPS will have SMAD4 mutations (14), while only 20% of patients with HHT will share this same SMAD4 mutation (6). Therefore, the incidence of JP-HHT syndrome as a result of the SMAD4 mutation is exceedingly rare.
Patients with JP often present in childhood with multiple hamartomatous (juvenile) polyps throughout the gastrointestinal tract (1, 2). These polyps are primarily found in the co-lorectum, but they can occur throughout the gastrointestinal tract from the stomach to rectum (2). The polyps are usually benign, but these patients have an increased risk of gastrointestinal cancer, ranging from 9% to 50%, which can involve the stomach, upper gastrointestinal tract, and pancreas (1, 2, 15). HHT involves vascular dysplasia in multiple organs including the skin, oral and nasal mucosa telangiectasias, and AVMs of the lungs, liver, brain, and gastrointestinal tract (16–18). Patients often experience excessive bleeding from the nose or gastrointestinal tract, with age-related penetrance (penetrance increases with age, 80% to 90% with recurrent epistaxis by age 21). Lung lesions can lead to hypoxemia, orthodeoxia, or hypocapnia and subsequent clubbing of the fingers and toes. Brain AVMs can cause strokes or spinal hemorrhage. Liver lesions can lead to high-output heart failure, portal hypertension, or biliary disease due to biliary ischemia (19). To date, the only treatment available for these severe hepatic forms of HHT is orthotopic liver transplantation; however, a recent study examined the efficacy of bevacizumab in severe hepatic forms of HHT associated with high cardiac output (20). They found that treatment with bevacizumab resulted in a decrease in cardiac output and reduced the duration and number of episodes of epistaxis. Thus, other options to liver transplantation may be available. Our patient had several characteristics of both JP and HHT, including nosebleeds, clubbing of the fingers and toes, and multiple hamartomatous polyps of the colon and small bowel, with a focal area of adenocarcinoma in her resected bowel.
Patients with JPS have a mutation in either the BMPR1A gene or SMAD4 gene, while patients with HHT have a mutation in either the ENG, ALK1, or SMAD4 genes or in another locus (21). SMAD4, the common gene in these two disorders, serves as a tumor suppressor gene by involvement in the transforming growth factor–β growth inhibitory pathway (22).
Our patient was referred for genetic testing for the SMAD4 mutation. Of interest, both her father and paternal grandfather had a history of colon polyps, AVMs, and nosebleeds (Figure (Figure1).1). This suggests that the patient's putative SMAD4 mutation was inherited paternally. Patients diagnosed with JPS or HHT may have hidden features of the other disorder. These undiscovered features often manifest themselves with extreme consequences. Therefore, any patient who has features of either JPS or HHT should have genetic screening for the SMAD4 mutation and be thoroughly examined for corresponding clinical pathology (6), so they can start the appropriate screening regimens. These include annual testing for anemia, screening for pulmonary AVMs by pulse oximetry every 1 to 2 years in the first decade of life and every 5 years by contrast echocardiogram thereafter, and antibiotic prophylaxis as a preventive measure for secondary complications if pulmonary AVMs are suspected for dental or invasive procedures, as well as a filter on intravenous lines. Additionally, screening with colonoscopy and upper endoscopy should begin by age 15, or earlier if symptomatic. If not, critical pathologies in the patient may be missed, resulting in unwanted patient outcomes. Finally, if the combined JP-HHT syndrome is known to exist in a family, targeted mutation testing specific to the proband's mutation is indicated for at-risk family members in the first two decades of life to implement the proper surveillance algorithms as described. The most cost-effective approach is to first test and identify the mutation in the proband, permitting targeted testing specific for the mutation in at-risk relatives. The targeted analysis is less expensive than the initial search for the mutation, which is most likely to be detected in the known affected family member. A genetic counseling referral can assist in educating the patient and family members regarding testing and implications. Moreover, the genetic counselor can serve as a liaison for referrals to the appropriate personnel for follow-up.
Acknowledgments
The authors thank Margaret Hinshelwood, PhD, manager of the Office of Scientific Publications for the Baylor Charles A. Sammons Cancer Center, for her editorial contributions.
References
Articles from Proceedings (Baylor University. Medical Center) are provided here courtesy of Baylor University Medical Center
Full text links
Read article at publisher's site: https://doi.org/10.1080/08998280.2012.11928877
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.1080/08998280.2012.11928877?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Article citations
Overlap syndrome of hereditary hemorrhagic telangiectasia and juvenile polyposis syndrome: ten years follow-up-case series and review of literature.
Fam Cancer, 24(1):1, 15 Nov 2024
Cited by: 0 articles | PMID: 39546055
Review
Hamartomatous polyps: Diagnosis, surveillance, and management.
World J Gastroenterol, 29(8):1304-1314, 01 Feb 2023
Cited by: 4 articles | PMID: 36925460 | PMCID: PMC10011967
Review Free full text in Europe PMC
A Rare Case of Hereditary Hemorrhagic Telangiectasia: A Case Report.
Cureus, 14(4):e24517, 27 Apr 2022
Cited by: 2 articles | PMID: 35651452 | PMCID: PMC9136551
[Hereditary hemorrhagic telangiectasia: 8 case report and lirerature review].
Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 35(11):1031-1034, 01 Nov 2021
Cited by: 0 articles | PMID: 34886610 | PMCID: PMC10128373
Case Report: Klippel-Trenaunay Syndrome - Recurrent Venous Thromboembolism and Vascular Malformation.
Int Med Case Rep J, 13:195-200, 21 May 2020
Cited by: 1 article | PMID: 32547256 | PMCID: PMC7247724
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The prevalence of hereditary hemorrhagic telangiectasia in juvenile polyposis syndrome.
Dis Colon Rectum, 55(8):886-892, 01 Aug 2012
Cited by: 32 articles | PMID: 22810475
Juvenile polyposis occurring in hereditary hemorrhagic telangiectasia.
Am J Med Sci, 317(1):59-62, 01 Jan 1999
Cited by: 14 articles | PMID: 9892274
Combined juvenile polyposis syndrome and hereditary hemorrhagic telangiectasia (JPS/HHT) with MRI and endoscopic correlation.
Clin Imaging, 54:37-39, 02 Dec 2018
Cited by: 1 article | PMID: 30521991
Hereditary hemorrhagic telangiectasia with juvenile polyposis--coincidence or linked autosomal dominant inheritance?
Z Gastroenterol, 37(5):385-388, 01 May 1999
Cited by: 10 articles | PMID: 10413846
Review


