Abstract
Study design
Markov model.Objective
Examine the 1-year effectiveness and cost-effectiveness (societal and payer perspectives) of adding nonpharmacologic interventions for chronic low back pain (CLBP) to usual care using a decision analytic model-based approach.Summary of background data
Treatment guidelines now recommend many safe and effective nonpharmacologic interventions for CLBP. However, little is known regarding their effectiveness in subpopulations (e.g., high-impact chronic pain patients), nor about their cost-effectiveness.Methods
The model included four health states: high-impact chronic pain (substantial activity limitations); no pain; and two others without activity limitations, but with higher (moderate-impact) or lower (low-impact) pain. We estimated intervention-specific transition probabilities for these health states using individual patient-level data from 10 large randomized trials covering 17 nonpharmacologic therapies. The model was run for nine 6-week cycles to approximate a 1-year time horizon. Quality-adjusted life-year weights were based on six-dimensional health state short form scores; healthcare costs were based on 2003 to 2015 Medical Expenditure Panel Survey data; and lost productivity costs used in the societal perspective were based on reported absenteeism. Results were generated for two target populations: (1) a typical baseline mix of patients with CLBP (25% low-impact, 35% moderate-impact, and 40% high-impact chronic pain) and (2) high-impact chronic pain patients.Results
From the societal perspective, all but two of the therapies were cost effective (<$50,000/quality-adjusted life-year) for a typical patient mix and most were cost saving. From the payer perspective fewer were cost saving, but the same number was cost-effective. Assuming all patients in the model have high-impact chronic pain increases the effectiveness and cost-effectiveness of most, but not all, therapies indicating that substantial benefits are possible in this subpopulation.Conclusion
Modeling leverages the evidence produced from clinical trials to provide more information than is available in the published studies. We recommend modeling for all existing studies of nonpharmacologic interventions for CLBP.Level of evidence
4.Free full text

Are Nonpharmacologic Interventions for Chronic Low Back Pain More Cost Effective than Usual Care? Proof of Concept Results from a Markov Model
Abstract
Study design.
Markov model.
Objective.
Examine the one-year effectiveness and cost-effectiveness (societal and payer perspectives) of adding nonpharmacologic interventions for chronic low back pain (CLBP) to usual care using a decision analytic model-based approach.
Summary of Background Data.
Treatment guidelines now recommend many safe and effective nonpharmacologic interventions for CLBP. However, little is known regarding their effectiveness in subpopulations (e.g., high-impact chronic pain patients), nor about their cost-effectiveness.
Methods.
The model included four health states: high-impact chronic pain (substantial activity limitations); no pain; and two others without activity limitations, but with higher (moderate-impact) or lower (low-impact) pain. We estimated intervention-specific transition probabilities for these health states using individual patient-level data from 10 large randomized trials covering 17 nonpharmacologic therapies. The model was run for nine 6-week cycles to approximate a 1-year time horizon. Quality-adjusted life-year (QALY) weights were based on six-dimensional health state short form (SF-6D) scores; healthcare costs were based on 2003–2015 Medical Expenditure Panel Survey data; and lost productivity costs used in the societal perspective were based on reported absenteeism. Results were generated for two target populations, 1) a typical baseline mix of patients with CLBP (25% low-impact, 35% moderate-impact and 40% high-impact chronic pain) and, 2) high-impact chronic pain patients.
Results.
From the societal perspective, all but two of the therapies were cost-effective (<$50,000/QALY) for a typical patient mix and most were cost saving. From the payer perspective fewer were cost saving, but the same number were cost-effective. Assuming all patients in the model have high-impact chronic pain increases the effectiveness and cost-effectiveness of most, but not all, therapies indicating that substantial benefits are possible in this subpopulation.
Conclusions.
Modeling leverages the evidence produced from clinical trials to provide more information than is available in the published studies. We recommend modeling for all existing studies of nonpharmacologic interventions for CLBP.
Introduction
Many safe and effective nonpharmacologic interventions for chronic low back pain (CLBP) are recommended in treatment guidelines.1–4 Recommended interventions include therapies such as acupuncture, mindfulness-based stress reduction, and yoga. Although these nonpharmacologic therapies have been available to the public for years, many are not part of the healthcare system, prescribed by physicians, nor generally covered by third-party payer plans. In addition, although all recommended therapies are effective, it is unknown whether some are more effective than others, especially for certain patient groups, and their impact on costs.
The effectiveness of each recommended nonpharmacologic therapy is supported by systematic reviews and meta-analyses over dozens of studies.3,5–7 In a few cases, these studies directly compare two or more of the recommended therapies, but usually each therapy is studied as adjunct to usual care and compared to usual care alone. Network meta-analysis (NMA, aka multiple or mixed treatment comparisons) is one technique that goes beyond traditional pairwise meta-analysis to allow comparisons and ranking between therapies that were not directly compared in clinical trials.8–11 However, the application of this technique requires that each therapy be directly compared to at least one comparator also used in another trial, thus, allowing indirect comparisons. Because of its common use, the logical common comparator for most therapies is usual care. However, the substantial variation across studies in what constitutes usual care and the lack of other common comparators lowers the reliability of NMA results.8
Decision analytic modeling is another method to synthesize evidence and compare across therapies.12,13 Modeling allows consistent inputs to be used across interventions, the simulation of experiments such as head-to-head trials, and the inclusion of economic outcomes even when they were not included in the original studies.12,14
Since each therapy is studied as an addition to usual care, its likely incremental effects in other healthcare settings can be estimated by comparing to the version of usual care used in the trial.
The 2016 US National Pain Strategy (NPS)15 placed a focus on those with high-impact chronic pain defined as that “associated with substantial restriction of participation in work, social, and self-care activities for six months or more.”15, p11 Studies have used different algorithms to identify those with high-impact chronic pain,16–21 and to demonstrate their significantly higher healthcare costs, lower quality of life, worse mood, and increased absenteeism.18–27 Nevertheless, studies of interventions for chronic pain still report results based on full samples—e.g., average change in symptoms or percent of patients who improve. To better understand chronic pain, better compare study results, and better target interventions, we need to move beyond simple duration of pain definitions (e.g., 3+ months) to identify meaningful chronic pain subtypes. Modeling can then be used to balance the baseline subtype mix of patients across studies and estimate differential treatment effects by subtype (e.g., chronic pain impact level).
Although several economic evaluations of nonpharmacologic therapies for CLBP have been published,28–34 because economic outcomes are not generalizable across settings,35 each study’s results can only provide useful information about cost impacts in its country and setting. Modeling is used to adjust inputs and assumptions to adapt study results to other settings.36
This study presents the results of a proof-of-concept modeling effort that utilized individual patient-level data from ten randomized studies and the categorization of these study’s participants by chronic pain impact level to estimate the effectiveness and cost-effectiveness of adding recommended nonpharmacologic therapies for CLBP to usual care.
Methods
This study used a Markov simulation model to compare the effectiveness and cost-effectiveness of a set of recommended nonpharmacologic therapies for CLBP. This modeling effort was part of a larger study examining the appropriateness of spinal mobilization and manipulation (M/M) for CLBP.37,38 This model was built to provide information to expert panels on the cost-effectiveness of M/M as compared to other nonsurgical interventions for CLBP to see if this information would change their original safety and effectiveness-based ratings of the appropriateness of M/M. To ensure face and construct validity39 and maximize the model’s usefulness and relevance,40 we built it with the input of two experts in the use of M/M for CLBP (ELH and HV) and a nine-member panel of expert representatives of the types of policy makers who could use the model’s results.
The model included transition probabilities that moved a hypothetical cohort of patients between four defined health states every 6 weeks for just over a year (Figure 1). One health state was defined as high-impact chronic pain (individuals with substantial activity limitations);15 one as a healthy, no pain state; and two others were without activity limitations, but with higher (moderate-impact) or lower (low-impact) pain. We estimated intervention-specific transition probabilities for these health states using individual patient-level data from the 10 trials41–50 covering 17 nonpharmacologic therapies. Trials of M/M and other common nonpharmacologic interventions for CLBP were chosen following these inclusion criteria: large sample sizes (e.g., at least 50 per arm), all (or most) of the subjects experienced CLBP (≥3 months’ duration), at least 6 months follow up, and US-, Canada- or UK-based for ease of acquisition and similar usual care. We also required that the study measured variables that were useful for the estimation of chronic pain impact levels—i.e., measures of pain, function and quality of life. Four trial datasets41–43,48 contained direct measure of chronic pain impact level using the Graded Chronic Pain Scale (GCPS):20,25,27,51,52 low-impact (Grade I), moderate-impact (Grade II), and high-impact (Grade III/IV) chronic pain. Chronic pain impact levels were estimated for the other datasets using logistic models with excellent predictive power53 using patients’ pain intensity, Roland-Morris Disability scores,51,54 and SF-1255 or SF-3656 items as independent variables. Details on the datasets acquired and the identification of each patient’s health state at baseline and over time are provided elsewhere.53
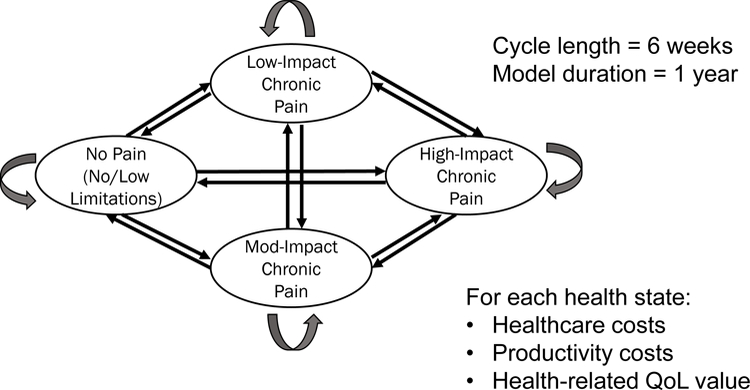
Schematic of the Markov model structure. Ovals represent the four health states: high-impact chronic pain (substantial activity limitations); no pain; and two without activity limitations, but with higher (moderate-impact) or lower (low-impact) pain.
Arrows represent the probability of transitions within and between health states and each therapy has its own set of transition probabilities. Patients begin in one of the three right-hand side health states and can transition to other health states in 6-week cycles.
Each health state was assigned a quality of life or quality-adjusted life-year (QALY) weight (calculated as the SF-6D57,58 score over 6 weeks) and 6-week costs (Table 1). The healthcare costs were estimated from 2003–2015 Medical Expenditure Panel Survey (MEPS, http://meps.ahrq.gov/mepsweb/) data,59 and represent average US-based 2015 healthcare costs associated with back pain. Estimates of absenteeism and utility were taken from trial data as described elsewhere.53 Productivity loss was estimated based on patient-reported absenteeism for those employed, and valued using the human capital approach60 at the US Bureau of Labor Statistics June 2015 national average employer cost of employee compensation for civilian workers of $33.19.61 The simulation tracked a cohort of hypothetical new CLBP patients over nine 6-week cycles and summed QALY gains and costs to yield estimated54-week health and cost outcomes.
Table 1.
Model inputs: 6-week healthcare costs (2015$) productivity loss (2015$), and utilities used to calculate quality-adjusted life-years (QALYs), Mean (SE)
| Health States | Back-Related Healthcare Costs1 | Productivity Loss (from Absenteeism)2 | Utilities (Measure of Health-Related Quality of Life)3 |
|---|---|---|---|
| No pain (no-to-low disability) | $ -- | $3.76 ($2.66) | 0.806 (0.004) |
| Low-impact chronic pain (low pain, low activity limitations) | $265.34 ($37.89) | $31.90 ($14.75) | 0.763 (0.003) |
| Moderate-impact chronic pain (high pain, low activity limitations) | $496.30 ($56.48) | $47.55 ($9.61) | 0.704 (0.006) |
| High-impact chronic pain (substantial activity limitations) | $689.91 ($54.38) | $288.93 ($51.88) | 0.610 (0.007) |
The resources used in the delivery of the nonpharmacologic interventions and usual care were captured from the information available in each study’s publications. The unit costs for each type of practitioner used were estimated as the average (after trimming the top and bottom 5%) of the costs per visit available in the MEPS. The full list of unit costs and their sources, the information available from each study, and the resulting treatment costs for each therapy are shown in Appendix.
Analysis
For each therapy, the model projected progression across health states based on estimated transition probabilities—i.e., the likelihood that an individual receiving that intervention will stay in their present state or move to another health state for the next 6-week cycle. Transition probabilities were estimated based on actual patient progression through health states in trial data. Nine trials measured outcomes over one year and the other50 went out 6 months. This study’s 6-month outcomes were held constant to 12 months based on the 6–12-month results of another yoga study.62
Because each study’s data collection occurred at different intervals, each study’s empirical transition probability matrix was first converted into a weekly matrix by translating the empirical probabilities into weekly rates and then converted into 6-week cycles that could be used in the model. We defined a typical mix of CLBP patients as having the following proportions at baseline: 25% low-impact, 35% moderate-impact, and 40% high-impact chronic pain. These proportions reflect the average baseline mix of patients in the trials used in the model.53
The Markov model was run for nine 6-week cycles to approximate a 1-year (54 week) time horizon. As individuals cycled through the health states they accumulated healthcare costs, productivity loss, and QALYs according to the values in Table 1. The model is probabilistic in that the effect of the uncertainty (SEs) of the mean input parameters, and of our estimation of the transition probabilities, was reflected in measures of output uncertainty (e.g., confidence intervals around 1-year costs and QALY gains).63 Because each intervention was studied as adjunct to usual care, incremental costs and effects for each therapy were calculated net of each study’s version of usual care. The model allows estimation of relative effectiveness and cost-effectiveness across all therapies. However, because these therapies are not equally available and accessible,64 and may not be equally acceptable to patients, our main results are presented for each therapy compared to usual care, and comparisons across all therapies are presented in the Appendix. Two studies did not include a usual care arm.48,65 For each we assigned two US-based usual care options based on the need for similar data collection schedules (see Appendix for details).
Finally, we performed tests of internal consistency (do model input changes have predictable effects on outputs) and external consistency (comparing model results to that shown in published studies).40 The Markov models were run using TreeAge Pro 2018 R2.1, Williamstown, MA, and other analyses used Microsoft Excel 2016, Redmond, WA.
Results
Table 2 gives the model’s incremental results compared to usual care for the societal and payer perspectives for a typical mix of chronic low-back pain patients. Table 3 shows the same information assuming all patients start with high-impact chronic pain. Figure 2 graphically displays the incremental point estimates for a typical mix of patients from the societal perspective. The 0,$0 point represents usual care and points below $50,000/QALY (a commonly defined threshold66) are generally considered cost-effective. As can be seen, all interventions but two (Traditional Chinese acupuncture and spinal manipulation assuming the same usual care) are cost-effective, many therapies have similar effectiveness and cost-effectiveness, and most showed cost savings from the societal perspective. The distance between the two versions of each intervention with assigned usual care arms indicates the sensitivity of interventions’ impacts to underlying usual care.
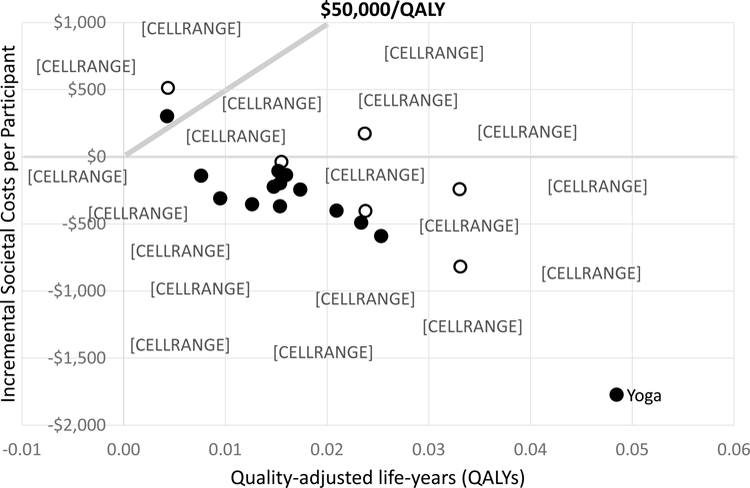
Effectiveness and cost-effectiveness from the societal perspective for 17 nonpharmacologic interventions compared to usual care alone for a typical* chronic low-back pain patient population.
Each intervention represented by a solid circle is compared to the usual care arm of its study. The three interventions identified with open circles came from two studies that did not include usual care arms. For these we assigned two US-based usual care arms from other studies. The usual care arms assigned for each are: (1) Usual care (Sherman); (2) Usual care (Moore); (3) Self-care education (Cherkin 2001); (4) Usual care (Cherkin 2009). Societal costs consist of three types of costs: the cost of the intervention itself, all other direct healthcare costs, and the indirect cost of productivity loss through absenteeism to employers. Incremental societal costs are these costs for each therapy minus the costs of usual care.
*A typical chronic low-back pain patient population was assumed to have 25% of patients with low-impact chronic pain, 35% with moderate-impact chronic pain, and 40% with high-impact chronic pain. These proportions roughly correspond to the average proportions seen in the studies included in the model.
Table 2.
One-Year Model Results for Each Nonpharmacologic Therapy Compared to Usual Care from the Societal and Payer Perspectives and Assuming a ‘Typical’* Mix of Chronic Low Back Pain Patients at Baseline
| Treatment Costs | Other Healthcare Costs (Societal) | Productivity Costs | Total Costs (Societal) | Total Costs (Payer)† | QALYs | |
|---|---|---|---|---|---|---|
| Active Trunk Exercise (1) | $1077 | −$898 | −$419 | −$241 (−1,060, 934) | $188 (−433, 993) | 0.033 (0.00, 0.06) |
| Active Trunk Exercise (2) | $1073 | −$536 | −$364 | $173 (−460, 1,042) | $530 (103, 1,073) | 0.024 (0.00, 0.04) |
| CBT Educational Program | $77 | −$286 | −$101 | −$310 (−1,040, 517) | −$197 (−692, 341) | 0.010 (−0.01, 0.03) |
| Chiropractic Care | $199 | −$252 | −$88 | −$142 (−1,655, 1,362) | −$40 (−1,012, 935) | 0.008 (−0.03, 0.04) |
| Exercise | $141 | −$284 | −$211 | −$354 (−696, 46) | −$146 (−369, 96) | 0.013 (0.00, 0.02) |
| Exercise + Manipulation | $509 | −$410 | −$235 | −$136 (−459, 257) | $117 (−84, 376) | 0.016 (0.01, 0.03) |
| Flexion Distraction (1) | $504 | −$906 | −$416 | −$818 (−1,557, 292) | −$389 (−929, 289) | 0.033 (0.01, 0.05) |
| Flexion Distraction (2) | $501 | −$544 | −$360 | −$403 (−941, 230) | −$47 (−415, 358) | 0.024 (0.01, 0.04) |
| Individualized Acupuncture | $480 | −$717 | −$355 | −$592 (−1,430, 367) | −$202 (−708, 496) | 0.025 (0.00, 0.05) |
| Manipulation | $480 | −$362 | −$225 | −$106 (−429, 282) | $120 (−100, 357) | 0.015 (0.01, 0.02) |
| Multidisciplinary Program | $260 | −$449 | −$181 | −$369 (−1,168, 563) | −$172 (−699, 450) | 0.015 (−0.01, 0.04) |
| Physical Therapy | $406 | −$439 | −$190 | −$223 (−1,777, 1,658) | −$13 (−1,108, 1,130) | 0.015 (−0.03, 0.05) |
| Relaxation Massage | $459 | −$592 | −$359 | −$491 (−1195, 431) | −$116 (−566, 529) | 0.023 (0.00, 0.04) |
| Spinal Manipulation (3) | $520 | −$13 | $6 | $513 (91, 888) | $457 (190, 726) | 0.004 (−0.01, 0.02) |
| Spinal Manipulation (4) | $520 | −$472 | −$85 | −$37 (−774, 689) | $47 (−382, 514) | 0.016 (0.00, 0.03) |
| Standardized Acupuncture | $487 | −$637 | −$252 | −$402 (−1,208, 447) | −$117 (−614, 481) | 0.021 (0.00, 0.04) |
| Structural Massage | $448 | −$406 | −$286 | −$244 (−978, 722) | $54 (−447, 658) | 0.017 (−0.01, 0.04) |
| TCM Acupuncture | $459 | −$139 | −$19 | $302 (−304, 875) | $328 (−83, 691) | 0.004 (−0.01, 0.02) |
| Therapeutic Massage | $437 | −$376 | −$258 | −$197 (−649, 296) | $69 (−202, 393) | 0.015 (0.00, 0.03) |
| Yoga | $385 | −$1626 | −$531 | −$1,773 (−2,730, −372) | −$1,136 (−1,784, −151) | 0.048 (0.02, 0.07) |
CBT = Cognitive behavioral therapy; QALY = Quality-adjusted Life-Year; TCM = Traditional Chinese acupuncture
Each intervention is compared to the usual care arm of their study with the exception of the two studies (three interventions) that did not include usual care arms. For these we assigned two of the other US-based usual care arms based on the closest matches in terms of data collection schedules. The usual care arms assigned for each are: (1) Usual care (Sherman); (2) Usual care (Moore); (3) Self-care education (Cherkin 2001); (4) Usual care (Cherkin 2009).
Table 3.
One-Year Model Results for Each Nonpharmacologic Therapy Compared to Usual Care from the Societal and Payer Perspectives and Assuming That All Chronic Low Back Pain Patients Have High-Impact Chronic Pain at Baseline
| Treatment Costs | Other Healthcare Costs (Societal) | Productivity Costs | Total Costs (Societal) | Total Costs (Payer)† | QALYs | |
|---|---|---|---|---|---|---|
| Active Trunk Exercise (1) | $1077 | −$1824 | −$915 | −$1662 (−2,977, 1,293) | −$707 (−1,499, 1,106) | 0.067 (−0.00, 0.11) |
| Active Trunk Exercise (2) | $1073 | −$655 | −$457 | −$39 (−1,241, 1,719) | $413 (−339, 1,446) | 0.029 (−0.01, 0.06) |
| CBT Educational Program | $77 | −$257 | −$89 | −$269 (−1,731, 1,434) | −$167 (−1,046, 488) | 0.008 (−0.04, 0.05) |
| Chiropractic Care | $199 | −$537 | −$263 | −$601 (−3,275, 2,420) | −$318 (−1,510, 1,411) | 0.019 (−0.06, 0.09) |
| Exercise | $141 | −$170 | −$208 | −$238 (−928, 505) | −$40 (−707, −5) | 0.010 (−0.01, 0.03) |
| Exercise + Manipulation | $520 | −$472 | −$354 | −$307 (−884, 419) | $54 (−456, 281) | 0.020 (0.00, 0.04) |
| Flexion Distraction (1) | $504 | −$1939 | −$980 | −$2,415 (−3,425, 341) | −$1,386 (−2,063, 389) | 0.072 (0.00, 0.10) |
| Flexion Distraction (2) | $501 | −$770 | −$522 | −$791 (−1,822, 359) | −$266 (−901, 466) | 0.033 (0.01, 0.06) |
| Individualized Acupuncture | $480 | −$1100 | −$651 | −$1,271 (−2,646, 439) | −$577 (−1,400, 380) | 0.042 (0.00, 0.08) |
| Manipulation | $480 | −$395 | −$339 | −$255 (−878, 430) | $83 (−492, 231) | 0.019 (0.00, 0.04) |
| Multidisciplinary Program | $260 | −$360 | −$145 | −$245 (−1,760, 1,237) | −$86 (−1,193, 653) | 0.012 (−0.02, 0.05) |
| Physical Therapy | $406 | −$979 | −$466 | −$1,039 (−4,351, 2,588) | −$525 (−1,849, 1,607) | 0.033 (−0.06, 0.18) |
| Relaxation Massage | $459 | −$722 | −$480 | −$744 (−1,938, 1,108) | −$250 (−1,218, 426) | 0.030 (−0.01, 0.06) |
| Spinal Manipulation (3) | $520 | −$451 | −$238 | −$168 (−1,174, 702) | $38 (−505, 634) | 0.021 (−0.00, 0.05) |
| Spinal Manipulation (4) | $520 | −$1172 | −$547 | −$1,199 (−2,269, 86) | −$630 (−966, 603) | 0.044 (0.01, 0.07) |
| Standardized Acupuncture | $487 | −$952 | −$450 | −$915 (−2,426, 873) | −$419 (−1,121, 611) | 0.033 (−0.01, 0.07) |
| Structural Massage | $448 | −$534 | −$422 | −$508 (−1,764, 1,166) | −$76 (−1,116, 702) | 0.024 (−0.02, 0.06) |
| TCM Acupuncture | $459 | $63 | $65 | $587 (−924, 1,823) | $517 (−268, 888) | −0.002 (−0.03, 0.03) |
| Therapeutic Massage | $437 | −$756 | −$468 | −$787 (−1,734, 296) | −$293 (−193, 381) | 0.029 (0.00, 0.06) |
| Yoga | $385 | −$2717 | −$1086 | −$3,419 (−4,930, −71) | −$2,174 (−2,370, −145) | 0.087 (0.01, 0.13) |
CBT = Cognitive behavioral therapy; QALY = Quality-adjusted Life-Year; TCM = Traditional Chinese acupuncture
Each intervention is compared to the usual care arm of their study with the exception of the two studies (three interventions) that did not include usual care arms. For these we assigned two of the other US-based usual care arms based on the closest matches in terms of data collection schedules. The usual care arms assigned for each are: (1) Usual care (Sherman); (2) Usual care (Moore); (3) Self-care education (Cherkin 2001); (4) Usual care (Cherkin 2009).
Yoga had the largest effects and cost savings. In fact, in terms of relative cost-effectiveness yoga was dominant—i.e., had lower costs and higher QALY gains than any other intervention (see Appendix). When costs are included from the payer perspective the same interventions are cost-effective, but fewer are cost saving.
When we assume all patients start with high-impact chronic pain, the results are more favorable for many therapies. However, some therapies have less favorable results: cognitive: cognitive behavioral therapy (CBT) educational program, chiropractic care, exercise, multidisciplinary care, and Traditional Chinese medicine (TCM) acupuncture, all had smaller QALY gains and higher costs in the high-impact group than with a typical mix of patients.
All internal consistency checks were successful. Model outputs responded as expected to variations in intervention costs, other healthcare costs, utilities, and transition probabilities. The Appendix contains the results of two tests of external consistency. Even though complete consistency is not expected since the model balanced baseline patient mixes and estimated QALYs, the model’s ranking of within-study functional effectiveness across arms was identical to that seen in the published studies. We also saw a consistent relationship between difference-in-difference estimates of function and incremental QALYs across studies.
Discussion
We present the results of a Markov model that estimates the effectiveness and cost-effectiveness of adding 17 nonpharmacologic interventions for CLBP to usual care. This model provides information beyond what is available from the individual studies, systematic reviews, and meta-analyses. The model estimates comparable results across studies by balancing baseline patient mixes (as would be expected with randomization), and using similar outcome measures (e.g., QALYs based on SF-6D, productivity costs and healthcare costs). Also, because we had patient-level data and were able to categorize patients into chronic pain impact levels to better track outcomes, and we were able to examine the effectiveness and cost-effectiveness of interventions assuming all patients start with one level of chronic pain. These results are useful to the better design and targeting of nonpharmacologic interventions for CLBP, and the use of a common set of US-based cost inputs provides US policy makers with initial estimates of economic impacts.
Our model’s health states were defined by chronic pain impact levels, and the results targeting high-impact chronic pain indicate that the biggest effects and costs for most interventions occur in these activity-limited patients. Given the number of other studies showing worse outcomes for patients with high-impact chronic pain—e.g., significantly higher healthcare costs, lower quality of life, worse mood, and more absenteeism18–27—it is not surprising that these patients would have a different response to treatment. However, this is the first study that examines how their response varies.
As with any Markov model, transition probabilities drive outcomes. Our transition probabilities were calculated from individual trial participants’ transitions through the health states they experienced over the trial year. Healthcare costs are related to patients’ health states, but health outcomes directly determine health states. Therefore, more confidence should be placed in the model’s estimates of QALY gains than of costs. One US cost-effectiveness analysis alongside a randomized trial of mindfulness-based stress reduction (MBSR) and group CBT for CLBP did find QALY gains in the range shown in model results.31 It also found that MBSR was cost saving from the societal and payer perspectives, and that CBT was cost neutral from the societal and likely cost-effective ($24,000/QALY) from the payer perspective, therefore, our cost estimates are reasonable. Nevertheless, modeling cost results should be interpreted with caution, considered more for their relative than absolute values, and replicated in relevant settings.
Another recent Markov model used three health states (chronic pain, improved pain, and death) to examine the relative effectiveness and cost-effectiveness of cognitive and mind-body therapies for CLBP.67,68 That model used 6-month cycles over 5 years, defined improved pain as ≥30% improvement in the RMDQ, and used other estimates for QALYs and healthcare costs as inputs. They concluded that yoga and MBSR offered high value for the money given their effectiveness and cost-effectiveness, acupuncture and CBT for pain offered intermediate value, and all four had evidence adequate to support coverage.
While we believe this model is useful, it is not without limitations. It now includes the results for 10 large randomized trials and 17 nonpharmacologic interventions for CLBP. Many other studies have been done that could be added to increase the information available, and the added studies might include therapies that are not as effective. This model in its present state should be considered a demonstration of how the results of different studies can be compared, and its results should not be used to make decisions. With more examples of each intervention, we could also examine the effect of any differences in protocols across studies. It is possible to use calibration to estimate transition probabilities from published studies when patient-level data are not available. However, confidence in these calibrated results would be lower. We used one measure of chronic pain impact levels to define our health states. There could be other health state definitions or other measures of chronic pain impact that would be more appropriate and better track effects and costs. To obtain estimates of the incremental effects for 3 interventions, we assigned usual care arms from those available from the other studies. We don’t know how close the assigned usual care arms are to the actual usual care underlying those studies, but the range in outcomes generated across the options assigned demonstrates the importance of including a usual care arm if a trial’s results are to be generalizable.
Markov modeling of nonpharmacologic interventions for CLBP is feasible and provides useful information about the effectiveness and cost-effectiveness of these interventions relative to usual care. According to model assumptions these interventions all improve health-related quality of life (QALYs) over usual care, and most, significantly so. In addition, most of these interventions appear cost-effective (and even cost saving) from the payer and societal perspectives, and many of the interventions have their largest impacts on those with high-impact chronic pain. Modeling leverages the investment made in existing trials to provide more useful information than is available from the published studies. We recommend this modeling effort be expanded to include data from all existing studies of nonpharmacologic interventions for chronic low back pain.
Supplementary Material
Supplemental Data File (doc., pdf., xls., etc.)
Acknowledgments:
The authors gratefully acknowledge all members of the extensive team that made this work possible including Nicholas Broten, Prodyumna Goutam, John Luke Irwin, Nima Shahidinia, and Howard Vernon. The authors would also like to acknowledge the following individuals and institutions who generously shared their data for this study: Dr. Jerilyn Cambron; Dr. Ram Gudavalli; Dr. Mitch Haas at the University of Western States; Dr. Eric Hurwitz; Drs. Daniel Cherkin, James E. Moore, Karen Sherman, and Michael Von Korff of Kaiser Permanente Washington Research Institute (formerly Group Health Research Institute); and the University of Warwick. Finally, the authors would like to thank the nine members of the panel of expert policy maker representatives, including Larry Becker, Rupali Das, Charles R Elder, R Lloyd Friesen, Christine M Goertz, and Anthony J Lisi.
The National Center for Complementary and Integrative Health (NICCIH) of the National Institutes of Health (NIH) (award number 1U19AT007912–01) funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
Contributor Information
Patricia M Herman, RAND Corporation, Santa Monica, CA.
Tara A Lavelle, Center for the Evaluation of Value and Risk in Health, Institute of Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA, and RAND Corporation, Boston, MA.
Melony E Sorbero, RAND Corporation, Pittsburgh, PA.
Eric L Hurwitz, Office of Public Health Studies, University of Hawaii, Mānoa, Honolulu, HI.
Ian D Coulter, RAND Corporation, Santa Monica, CA.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/brs.0000000000003097
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6779140
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/67698329
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/brs.0000000000003097
Article citations
[DNVF Memorandum: Objectives and Methods of Physical Activity-Related Health Services Research].
Gesundheitswesen, 24 Jul 2024
Cited by: 0 articles | PMID: 39047784 | PMCID: PMC11465437
Economic Evaluation of Digital Therapeutic Care Apps for Unsupervised Treatment of Low Back Pain: Monte Carlo Simulation.
JMIR Mhealth Uhealth, 11:e44585, 29 Jun 2023
Cited by: 3 articles | PMID: 37384379 | PMCID: PMC10365619
Reducing the burden of low back pain: results from a new microsimulation model.
BMC Musculoskelet Disord, 23(1):804, 23 Aug 2022
Cited by: 0 articles | PMID: 35996103 | PMCID: PMC9396830
CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022.
MMWR Recomm Rep, 71(3):1-95, 04 Nov 2022
Cited by: 321 articles | PMID: 36327391 | PMCID: PMC9639433
A cross-sectional study to validate an administrative back pain severity classification tool based on the graded chronic pain scale.
Sci Rep, 12(1):16927, 08 Oct 2022
Cited by: 0 articles | PMID: 36209228 | PMCID: PMC9547910
Go to all (13) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Update of Markov Model on the Cost-effectiveness of Nonpharmacologic Interventions for Chronic Low Back Pain Compared to Usual Care.
Spine (Phila Pa 1976), 45(19):1383-1385, 01 Oct 2020
Cited by: 6 articles | PMID: 32516169 | PMCID: PMC7751339
Cost-effectiveness of Mindfulness-based Stress Reduction Versus Cognitive Behavioral Therapy or Usual Care Among Adults With Chronic Low Back Pain.
Spine (Phila Pa 1976), 42(20):1511-1520, 01 Oct 2017
Cited by: 36 articles | PMID: 28742756 | PMCID: PMC5694631
Effectiveness, costs and cost-effectiveness of chiropractic care and physiotherapy compared with information and advice in the treatment of non-specific chronic low back pain: study protocol for a randomised controlled trial.
Trials, 18(1):613, 22 Dec 2017
Cited by: 3 articles | PMID: 29273083 | PMCID: PMC5741874
Cost-Effectiveness Data Regarding Spinal Cord Stimulation for Low Back Pain.
Spine (Phila Pa 1976), 42 Suppl 14:S72-S79, 01 Jul 2017
Cited by: 15 articles | PMID: 28399549
Review

