Advertisement
Research Article Free access | 10.1172/JCI65459
Th9 cells promote antitumor immune responses in vivo
Yong Lu, Sungyoul Hong, Haiyan Li, Jungsun Park, Bangxing Hong, Lijuan Wang, Yuhuan Zheng, Zhiqiang Liu, Jingda Xu, Jin He, Jing Yang, Jianfei Qian, and Qing Yi
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Lu, Y. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Hong, S. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Li, H. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Park, J. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Hong, B. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Wang, L. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Zheng, Y. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Liu, Z. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Xu, J. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by He, J. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Yang, J. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Qian, J. in: JCI | PubMed | Google Scholar
Department of Lymphoma and Myeloma, Division of Cancer Medicine, and Center for Cancer Immunology Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Find articles by Yi, Q. in: JCI | PubMed | Google Scholar
Authorship note: Yong Lu and Sungyoul Hong contributed equally to this work.
Published October 15, 2012 - More info
J Clin Invest. 2012;122(11):4160–4171. https://doi.org/10.1172/JCI65459.
© 2012 The American Society for Clinical Investigation
Received: June 21, 2012; Accepted: August 23, 2012
Related article:
Abstract
The incidence of melanoma — the most aggressive form of skin cancer — is dramatically increasing, while the development of innovative therapeutic strategies continues to be challenging, especially due to a lack of knowledge about the molecular mechanisms underlying melanoma progression as well as antitumor immunity. In this issue of the JCI, Yong Lu and colleagues report a central role for Th9 cells in antitumor immunity.
Authors
Edgar Schmitt, Tobias Bopp
-
Abstract
Th9 cells are a subset of CD4+ Th cells that produce the pleiotropic cytokine IL-9. IL-9/Th9 can function as both positive and negative regulators of immune response, but the role of IL-9/Th9 in tumor immunity is unknown. We examined the role of IL-9/Th9 in a model of pulmonary melanoma in mice. Lack of IL-9 enhanced tumor growth, while tumor-specific Th9 cell treatment promoted stronger antitumor responses in both prophylactic and therapeutic models. Th9 cells also elicited strong host antitumor CD8+ CTL responses by promoting Ccl20/Ccr6-dependent recruitment of DCs to the tumor tissues. Subsequent tumor antigen delivery to the draining LN resulted in CD8+ T cell priming. In agreement with this model, Ccr6 deficiency abrogated the Th9 cell–mediated antitumor response. Our data suggest a distinct role for tumor-specific Th9 cells in provoking CD8+ CTL-mediated antitumor immunity and indicate that Th9 cell–based cancer immunotherapy may be a promising therapeutic approach.
-
Introduction
Identification of CD4+ Th subsets has improved our understanding of adaptive immunity. Based on their cytokine secretion and immune regulatory function, Th cells include, but are not limited to, Th1, Th2, and Th17 (1). The roles of CD4+ Th subsets in antitumor immunity remain controversial and have not been comprehensively examined (2, 3). Th1 cells, secreting IFN-γ and capable of enhancing activity of CD8+ CTLs, have traditionally been considered the most efficient CD4+ T cell subset to generate antitumor immunity (4, 5). Th2 cells are known to promote the antitumor immune response by recruitment of tumoricidal eosinophils and macrophages into the tumor microenvironment (6), but are also known to inhibit cell-mediated immunity by secreting IL-4 and IL-10 to promote tumor progression (7). The role of Th17 in malignancy is currently under debate (8). The Th17 lineage can promote tumor growth by upregulating prosurvival and pro-angiogenic genes (9, 10), while more recently, Th17-polarized cells have also been shown to better mediate destruction of B16 melanoma than Th1 cells (11–14). This Th17-mediated antitumor effect is critically dependent on conversion of Th17 to Th1 cells and/or recruitment of other arms of the immune system, and IL-17A only marginally or partially contributes to this effect.
Recently, there has been renewed interest in IL-9–producing CD4+ T cells, including Th2 and Th17 cells and Tregs (15, 16). IL-9 production was first associated with the Th2 phenotype and plays an important role in the pathogenesis of asthma, IgE class switch recombination, and resistance to parasites (17–19). However, IL-4, a critical Th2 inducer, has a minimal effect on IL-9 expression during naive T cell differentiation and, together with TGF-β, greatly enhances IL-9 production and inhibits the production of classical Th2 cytokines (20–22). The most consistent IL-9–producing T cells generated with the cytokines TGF-β and IL-4 are characterized as an additional Th subset and termed Th9 to distinguish them from classical Th2 cells (16, 23). Th9 and IL-9 cells are pro-inflammatory, and appear to function in a broad spectrum of autoimmune diseases and allergic inflammation (16, 17, 24, 25). On the other hand, IL-9 has also been reported to promote the maintenance of tolerant environment by enhancing both Tregs and mast cell–mediated immunosuppressive functions (26–28), and by limiting the pathogenic activity of Th17 (29). In addition, IL-9 may lead to decreased production of IL-12 and limit the capacity of APCs to induce a Th1-type immune response (30). These observations indicate that IL-9 is a pleiotropic cytokine and functions as both a positive and negative regulator of immune responses. Nevertheless, the role of Th9 and IL-9 cells in cancer immunity is unknown, and their ability to cause inflammation and destruction of tissues might be of interest in the therapy of malignancy.
In the current study, we first analyzed the effect of IL-9 neutralization in the poorly immunogenic B16 melanoma lung model. In addition, tumor-specific Th9 cells were transferred into mice in several prophylactic and therapeutic tumor models. Our results suggest that IL-9 and Th9 protect mice from tumor development. Interestingly, tumor-specific Th9 cells promote strong CD8+ CTL activation by recruitment of DCs into tumor tissues and subsequent presentation of tumor antigens in tumor-draining LNs (TDLNs). Ccl20 expression in tumor tissues induced by Th9 cells largely contributes to the antitumor effects. Therefore, we describe for the first time that Th9 cells induce a protective antitumor immunity by eliciting a tumor-specific CTL response.
-
Results
Neutralization of IL-9 in mice promotes tumor growth. To investigate the role of IL-9 in modulating tumor growth in vivo, we first treated C57BL/6 mice with IL-9–neutralizing antibodies (α–IL-9) and then challenged the mice with B16 melanoma by i.v. injection. We assessed lung metastasis on day 18 after tumor challenge. Mice treated with α–IL-9 developed increased tumor foci and multiple tumor fusions as compared with control IgG-treated mice (Figure 1A). Lung weight of mice treated with α–IL-9 also significantly increased, indicating larger tumor burden in the lungs as compared with control IgG-treated mice (Figure 1A).
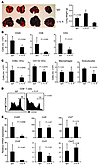 Figure 1
Figure 1IL-9–neutralized mice are more susceptible to developing lung melanoma. C57BL/6 mice (n = 4–5/group) receiving control IgG or α–IL-9 every other day beginning 1 day before i.v. challenge of 1 × 105 B16 melanoma cells were analyzed on day 18 after challenge. The P values in the graphs show comparisons between IgG and α–IL-9 groups. (A) Images and weights of lungs show increased tumor development in the lungs of mice treated with α–IL-9. NT, untreated. (B) Number of total leukocytes, CD8+ T cells, and CD4+ T cells in the lung leukocyte fraction analyzed with FACS. (C) Cell numbers of myeloid population subsets in the lung leukocyte fractions analyzed by FACS. (D) Expression of CD44 on CD8+ T cells from the lung. Numbers above scale bars indicate percentage of CD44hi cells. (E) RT-PCR analysis of mRNA expression of chemokines and their receptors in the lung tumor tissues. Data shown were normalized to the β-actin gene. Representative results from 1 of 2 performed experiments are shown.
We observed significantly decreased numbers of CD45+ leukocytes in the lung tissues of the mice treated with α–IL-9, as well as substantially decreased leukocyte subsets including CD4+ T cells, CD8+ T cells, CD8α+ DCs, and CD11b+ DCs in the lung tissues, as compared with control IgG-treated mice (Figure 1, B and C). In contrast, there was no significant difference in the numbers of CD11b+F4/80+ macrophages and Gr1+ granulocytes between these 2 groups. Additionally, we found that CD8+ T cells from the lung tissues of mice neutralized with α–IL-9 had decreased expression of CD44, indicating that these T cells were less activated (Figure 1D). These data suggested that tumor growth was enhanced in the absence of IL-9, which was associated with a decreased infiltration of leukocytes and decreased activation of CD8+ T cells in the tumor tissue.
This decreased infiltration of leukocytes in the absence of IL-9 led us to investigate whether IL-9 regulated chemokine expression in the tumor tissues by analyzing mRNA expression. We found that the total lung tissues from the mice treated with α–IL-9 had sharply decreased mRNA expression of Ccl20 and its receptor Ccr6, while the change in expression of other chemokines and their receptors was not significant (Figure 1E). Our data thus far indicated that IL-9 favored tumor protection, possibly through Ccl20-mediated leukocyte recruitment into the tumor tissue.
Tumor-specific Th9 cells protect mice against tumor development. To better understand the role of Th9 cells in tumor immunity, we used an OVA-expressing B16 cell line (B16-OVA) in tumor models and generated OVA-specific Th9 cells in vitro. These differentiated Th9 cells used in each experiment typically contained about 25% IL-9– and 10% IL-10–expressing CD4+ T cells, with low expression of IFN-γ, IL-4, and IL-17, as evaluated by intracellular cytokine staining (ICS) and ELISA (Figure 2A and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI65459DS1). We first transferred Th9 cells into C57BL/6 mice on the same day when B16-OVA was injected i.v. These mice treated with Th9 cells developed significantly reduced numbers of lung tumor foci on day 19 as compared with control mice receiving PBS (Figure 2B). This antitumor protection in Th9-treated mice was associated with a significantly increased CD45+ leukocyte infiltration in lung tumor tissues and with substantially increased leukocyte subsets, including CD4+ T cells, CD8+ T cells, CD8α+ DCs, and CD11b+ DCs, as compared with mice receiving only PBS, whereas the numbers of macrophages and granulocytes did not differ between the groups (Figure 2, C and D). In addition, lung tumor tissue-infiltrating CD4+ and CD8+ T cells in Th9-treated mice had significantly upregulated expression of CD44 compared with PBS-treated mice (Figure 2E). The Th9 cell–induced inflammatory response was also related to substantially increased mRNA expression of chemokine Ccl20 and its receptor Ccr6 in tumor, whereas other chemokines and their receptors tested were not significantly changed (Figure 2F). Taken together, these results suggest that Th9 cells transferred in vivo might induce lung tumor tissues to produce Ccl20, which promotes the recruitment of DCs and activated T cells into the tumor tissues for the destruction of tumor cells.
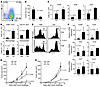 Figure 2
Figure 2Prophylactic effect of tumor-specific Th9 cells. (A) OVA-specific Th9 cells used in each experiment typically contained about 25% IL-9–expressing CD4+ OT-II T cells. (B–F) C57BL/6 mice (n = 4–5/group) received PBS or 3 × 106 Τh9 cells on the same day when mice were i.v. challenged with 1 × 105 B16-OVA cells, and were analyzed on day 19 after challenge. (B) Comparison of tumor foci numbers in the lung between PBS- and Th9 cell–treated mice. (C) Number of total leukocytes, CD8+ T cells, and CD4+ T cells in the lung leukocyte fraction analyzed by FACS. (D) Cell numbers of myeloid population subsets in the lung leukocyte fraction analyzed by FACS. (E) Expression of CD44 on CD4+ and CD8+ T cells from the lung. (F) RT-PCR analysis of mRNA expression of chemokines and their receptors in lung tumor tissues. Data shown were normalized to the β-actin gene. (G) Th1 or Th9 cells (2 × 106) were s.c. injected on the same day and at the same location where 5 × 105 B16-OVA tumor cells had been implanted s.c. Tumor growth curves are shown. P = 0.0033 for Th1 versus Th9 on day 21. (H) Th1 or Th9 cells (3 × 106) were i.v. transferred on the same day that 2 × 105 B16-OVA tumor cells were s.c. injected. Tumor growth curves are shown. P = 0.012 for Th1 versus Th9 on day 21. Representative results from 1 of 3 repeated experiments are shown. The P values in the graphs show comparisons with PBS.
Importantly, Tregs have been demonstrated to have Ccr6-dependent homing into tumor sites, and their function may be increased in the presence of IL-9 (26, 31). We also detected a significantly increased number of Tregs, as well as a higher ratio of Tregs to total CD4+ T cells, in the lung of Th9 cell–treated tumor-bearing mice as compared with PBS-treated tumor-bearing mice (Supplemental Figure 2, A and B). However, the ratio of Tregs to CD8+ T cells was sharply decreased in Th9 cell-treated mice (Supplemental Figure 2B), indicating that Th9 cell treatment preferentially recruited effector CD8+ T cells into the tumor microenvironment. In addition, mast cells might be another potential cell population that can be activated upon Th9 cell treatment (28). However, we could not detect activated MCPT7+ mast cells in any lung sections of the mice (data not shown) and found only about 10 mast cells in lung sections of PBS and Th9 cell–treated mice using toluidine blue stain (Supplemental Figure 2C), suggesting that mast cells may not be important for Th9 cell–mediated tumor rejection in our study.
To investigate whether the protection of tumor-specific Th9 cells was limited to tumor located in the lung, we used the s.c. B16-OVA model. OVA-specific Th1 or Th9 cells were injected s.c. on the same day and at the same location where the B16-OVA tumor cells were implanted (Figure 2G). Th1 cell treatment reduced tumor growth, but to a lesser extent compared with Th9 cells, which eliminated the tumor burden. We also injected Th1 or Th9 cells i.v. to the mice on the same day when B16-OVA cells were implanted s.c. (Figure 2H). Adoptive transfer of Th1 cells did not protect against tumor development, but Th9 cells substantially reduced the growth of B16-OVA under the skin. These results indicate that tumor-specific Th9 cells can elicit strong antitumor immunity in different organ locations in prophylactic models.
Therapeutic effects of tumor-specific Th9 cells against established tumors. Next we tested whether Th9 and Th1 cell adoptive transfer can help eradicate 5-day established lung tumor metastasis. Our results show that mice treated with Th1 cells had about 50% fewer tumor foci, whereas mice treated with Th9 cells had an approximately 75% reduction in lung tumor foci compared with mice receiving PBS (Figure 3A and Supplemental Figure 3). Surprisingly, Th9 cells had significantly greater antitumor potency than Th1 cells in this therapeutic setting.
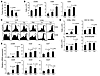 Figure 3
Figure 3Therapeutic effect of tumor-specific Th9 cells. PBS or 3 × 106 Th1 or Th9 cells were transferred i.v. to C57BL/6 mice bearing 5-day established pulmonary B16-OVA melanoma. Mice (n = 4–5/group) were analyzed on day 19 after challenge. (A) Comparison of tumor foci numbers in the lung between treated mice. P = 0.042, Th1 versus Th9. (B) Number of total leukocytes, CD8+ T cells, and CD4+ T cells in the lung leukocyte fraction analyzed by FACS. P = 0.0014, Th1 versus Th9 for CD8+ T cells. (C) Expression of CD44 on CD4+ and CD8+ T cells from the LLN and lung leukocyte fraction. For Th1 versus Th9, P = 0.011 (LLN CD4+ T cells), P = 0.022 (LLN CD8+ T cells), P = 0.020 (lung CD4+ T cells), and P = 0.0035 (lung CD8+ T cells). (D) Cell numbers of myeloid population subsets in the lung leukocyte fraction analyzed by FACS. P = 0.00031, Th1 versus Th9 for CD8α+ DCs. (E) RT-PCR analysis of mRNA expression of chemokines and their receptors in the lung tumor tissues. Data shown were normalized to β-actin gene. For Th1 versus Th9, P = 0.014 (Ccl20), P = 0.025 (Ccr6). Representative results from 1 of 3 repeated experiments are shown. P values in the graphs show comparisons with PBS.
A further analysis of the inflammatory status of the lung tumor tissues revealed that Th1 cell treatment only resulted in slightly increased infiltration of CD45+ leukocytes, CD4+ T cells, CD8+ T cells, CD8α+ DCs, and CD11b+ DCs, whereas Th9 cell–treated mice had significantly increased numbers of all these infiltrating cells, as compared with mice receiving PBS (Figure 3, B and D). Both Th1 cell– and Th9 cell–transferred mice had higher percentages of activated CD4+ and CD8+ T cells, shown by the CD44 expression, in the lung as compared with PBS-treated mice, while there was no significant difference in the numbers of macrophages and granulocytes among these 3 groups (Figure 3, C and D).
Results from mRNA expression of chemokines and their receptors consistently revealed that only Th9 cell treatment substantially induced the expression of Ccl20 in tumor tissues and selective recruitment of Ccr6+ cells in the lung as shown by the significantly increased Ccr6 mRNA expression (Figure 3E). In Th1 cell–treated mice, mRNA expression of receptors including Ccr6, Ccr2, and Cxcl1, were slightly but not significantly increased in the lung tumor tissues as compared with PBS-treated mice (Figure 3E). These data together indicated that Th9 cells induced different antitumor inflammatory responses in the tumor tissues as compared with Th1 cells, which might be due to Th9 cell–induced expression of Ccl20 in the tumor tissues.
By using ELISA, we confirmed that lung tumor tissues of Th9 cell–treated mice produced Ccl20 (Supplemental Figure 4A). However, Th0, Th1, and Th9 cells did not produce a significant amount of the chemokine (Supplemental Figure 4B). We isolated lung epithelial cells and CD45+ lung-infiltrating leukocytes from PBS or Th9 cell–treated tumor-bearing mice and, by using both RT-PCR and ELISA, showed that lung epithelial cells but not leukocytes produced a high amount of Ccl20 in Th9 cell–treated mice (Supplemental Figure 4, C and D). Notably, lung epithelial cells but not leukocytes or B16-OVA tumor cells produced Ccl20 in response to IL-9 stimulation in a dose-dependent manner (Supplemental Figure 4E). In addition, we also detected Il9r mRNA expression in lung epithelial cells, whereas B16-OVA cells neither expressed Il9r mRNA nor exhibited different proliferation rate in response to IL-9 stimulation (Supplemental Figure 4, F and G). Finally, by using immunohistochemistry staining, we further confirmed that the main sources of Ccl20 production were bronchial and alveolar epithelial cells but not the tumor cells in Th9 cell–treated lung tissues (Supplemental Figure 5).
Th9 cells retain their cytokine expression profiles in vivo. Th9 cells have been reported to be genetically unstable and flexible in cytokine production, based on the observation that Th9 cells recovered from T cell transfer models of both colitis and EAE produce both IL-17 and IFN-γ following in vitro PMA/ionomycin stimulation (22, 32). To determine whether Th9 cells, similar to Th17 cells, might have a greater plasticity compared with Th1 cells in tumor models, we tested the cytokine production profile of Th9 cells transferred in vivo. We first transferred Th1 and Th9 cells to mice (wild-type and Ifng–/– mice) bearing 5-day established pulmonary melanoma, and harvested and restimulated lung LN (LLN) cells and total lung cells with OVA peptides for 36 hours. Supernatants were tested for cytokine production using ELISA (Figure 4A). Our results showed that in both the LLN and the lung, there was substantially upregulated IL-9 and IL-10 production in Th9-transferred mice (both wild-type and Ifng–/– mice). IFN-γ production was detected in LLN cell cultures from both Th1 cell– and Th9 cell–transferred wild-type mice, although IFN-γ production from Th1 cell–transferred mice was higher than that of Th9 cell–transferred mice. Interestingly, IFN-γ production was not detected in lung cells of mice (both wild-type and Ifng–/– mice) transferred with Th1 cells, indicating that Th1 cells were not recruited to the tumor tissues, at least by day 4 following injection. Notably, IFN-γ production was detected in lung cells of the mice transferred with Th9 cells in the wild-type mice, but IFN-γ production from cells of both the LLN and lung was very low or undetected in Ifng–/– mice, indicating that IFN-γ production was derived from the host cells other than the transferred Th9 cells. In addition, IL-17 production could only be detected from lung cell cultures of mice transferred with Th9 cells, while LLN cells from mice with different treatments produced low but similar levels of IL-17, suggesting that Th9 cell treatment also recruited IL-17–producing cells to the tumor tissues. Cytokine production from tumor-bearing, PBS-treated wild-type (Figure 4A) and Ifng–/– (data not shown) mice was similar, while IL-4 production was low or undetected in all the supernatants tested. We also detected significantly increased TNF-α production in both the lung and LLN of Th9 cell–treated wild-type mice. However, other Th2-related cytokines such as IL-5 and IL-13 were not detected in Th9 cell–treated mice (Supplemental Figure 6A).
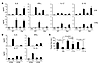 Figure 4
Figure 4Th9 cells maintain cytokine expression profile in tumor-bearing mice. (A) PBS or 3 × 106 Th1 or Th9 cells were i.v. transferred to mice (wild-type and Ifng–/– mice) with 5-day established pulmonary B16-OVA melanoma. On day 4 after transfer, total LLN and lung cells were harvested and restimulated with OVA peptides for 36 hours. Cytokine levels in culture supernatants were tested using ELISA. (B) OVA-specific CFSE-labeled Th1 or Th9 cells (3 × 106) were i.v. transferred into mice with 5-day pulmonary B16-OVA melanoma. On day 4 after transfer, CFSE+ Th1 or Th9 cells and CFSE– endogenous cells were sorted from LLNs with flow cytometer. CFSE+ Th1 or Th9 cells were maintained with 10 U/ml IL-2, and CFSE– cells were restimulated with 5 μg/ml OVA323–339 and OVA257–264 peptides for 36 hours. Cytokine levels in the supernatant were tested using ELISA. (C) mAbs neutralizing IL-9, IL-10, or control IgG were i.p. injected to C57BL/6 wild-type mice bearing 5-day established pulmonary B16-OVA melanoma; some groups of mice were also i.v. transferred with 3 × 106 Th9 cells. The graph shows the comparison of the numbers of tumor foci in the lungs of mice (n = 4/group) receiving the indicated treatments. Representative results from 1 of 2 performed experiments are shown.
To further demonstrate that Th9 cells maintained their phenotype in vivo, we labeled tumor-specific Th1 and Th9 cells with CFSE and transferred them to tumor-bearing mice. On day 4 after the transfer, mice were sacrificed and CFSE+ cells were sorted out from LLNs and stimulated with IL-2 for 36 hours, while CFSE– cells were stimulated with OVA peptides. Supernatants were tested with ELISA for cytokine production (Figure 4B and Supplemental Figure 6B). CFSE+ Th9 cells retained production of IL-9 and IL-10, with very low amounts of IFN-γ, TNF-α, and IL-17, and CFSE+ Th1 cells maintained the production of IFN-γ. Peptide-stimulated IFN-γ and TNF-α production was also detected in CFSE– cells from mice transferred with Th1 or Th9 cells, indicating an induction of tumor-specific Th1 response in those mice. Therefore, we concluded that transferred Th9 cells maintained their IL-9 and IL-10 cytokine production and did not convert to Th1 or Th17 phenotypes.
Moreover, we i.p. injected α–IL-9 and α-IL-10 neutralizing antibodies every other day, beginning 1 day before Th9 cells were transferred to tumor-bearing mice. We found that blockade of IL-9 but not IL-10 largely abrogated tumor protection mediated by Th9 cell transfer (Figure 4C). Our results thus indicated that Th9 cell–mediated tumor protection was highly dependent on IL-9 production by Th9 cells.
Th9 cells promote a strong tumor-specific CD8+ CTL response. Unlike Th1 cells, tumor-specific Th9 cells did not have any cytotoxic activity against B16-OVA cells in vitro (data not shown), although Th9 cells mediated the destruction of B16-OVA cells in vivo. Since we detected significantly increased CD8+ T cells in lung tumor tissues, and CD8+ T cells are known effectors that eliminate cancer cells by direct cytotoxicity, we hypothesized that Th9 cells may induce the activation and/or recruitment of tumor-specific CD8+ CTLs. To test our hypothesis, we enumerated tetramer-specific CD8+ T cells recognizing SIINFEKL peptide derived from OVA (OVA-tetramer+CD8+ CTLs). In Th9 cell–treated mice, there were higher percentages and larger numbers of OVA-tetramer+CD8+ CTLs in tumor tissues, constituting about 30% of total lung CD8+ T cells (Figure 5A) and 13% of total tumor-infiltrating leukocytes when Th9 cells were s.c. injected at the tumor-injection site (Figure 5B), or 5% of total tumor-infiltrating leukocytes when Th9 cells were adoptively transferred i.v. (Figure 5C). Such an OVA-tetramer+CD8+ CTL population was almost absent in PBS-treated mice and only slightly increased in Th1 cell–treated mice (Figure 5, A–C). In addition, there was a significant increase in the number of granzyme B–producing CD8+ T cells in response to OVA-peptide restimulation in the tumor tissues of Th9 cell–treated mice (Figure 5, A and B). Most importantly, Th9 cell–mediated protection in vivo against tumor was almost completely abrogated when CD8+ T cells were depleted by i.p. injection of α-CD8 antibodies every 3 days starting from day 1 before Th9 cell transfer (Figure 5D).
 Figure 5
Figure 5Th9 cells promote tumor-specific CD8+ CTL response in tumor-bearing mice. (A–C) FACS analysis of the frequencies of CD8+ T cells staining positive for tumor infiltrating OVA tetramer (Kb-SIINFEKL), relative to total CD8+ T cells, (A) in therapeutic lung models, (B) in the total leukocytes in prophylactic s.c. tumor models when T cells were s.c. injected, or (C) in the total leukocytes in prophylactic s.c. tumor models when T cells were i.v. transferred. Graphs show the total number of tumor-infiltrating OVA tetramer–positive CD8+ T cells and/or granzyme B–producing CD8+ T cells in the leukocyte fraction. n = 4 mice/group. (D) PBS or 3 × 106 Th1 or Th9 cells were i.v. transferred into mice bearing 5-day established pulmonary B16-OVA melanoma. A group of mice transferred with Th9 cells also received depleting mAbs against CD8 every 3 days starting from 1 day before T cell transfer. Shown are the lung foci numbers observed on day 19 after challenge (n = 4 mice/group). Representative results from 1 of 2 performed experiments are shown. In D, P values indicate comparisons with PBS.
To further confirm an induction of tumor-specific CD8+ CTL response by adoptively transferred Th9 cells in vivo, we labeled OT-I CD8+ T cells with CFSE and adoptively transferred them to mice bearing 5-day established pulmonary B16-OVA, or to mice bearing s.c. tumors on the same day when mice were treated with PBS, Th1, or Th9 cells. On day 3 after the OT-I cell transfer, we found significantly increased numbers of CFSE+ OT-I cells and CFSEloIFN-γ+ OT-I cells in TDLNs of mice receiving Th9 cell transfer (Figure 6A) or s.c. injection (Figure 6B) as compared with mice receiving PBS or Th1 cell treatment. More importantly, the presence of substantially upregulated, granzyme B–producing OT-I cells were demonstrated (Figure 6, C and D). We also detected, on day 3 after OT-I cell transfer, significantly increased numbers of CFSE+ OT-I cells in the lung tumor tissues of Th9 cell–treated mice as compared with PBS or Th1 cell–treated mice (Supplemental Figure 7). Since CD8+ T cells represented the major population recruited into the tumor microenvironment by Th9 cell treatment, possibly via Ccl20, we examined and confirmed the surface expression of Ccr6 by infiltrating CD8+ T cells in the lung tumor tissue of Th9 cell–treated mice (Supplemental Figure 8). These results thus far suggest that Th9 cells provided “help” to elicit an endogenous tumor-specific CD8+ CTL response in vivo, possibly by favoring tumor antigen priming of CD8+ CTLs in TDLNs and also by helping their subsequent migration to the tumor sites.
 Figure 6
Figure 6Th9 cell treatment enhances tumor-specific CD8+ CTL differentiation. (A and C) CFSE-labeled OT-I CD8+ T cells (3 × 106) were i.v. transferred into mice bearing 5-day pulmonary B16-OVA melanoma, which were also i.v. transferred at the same day with 3 × 106 Th1 or Th9 cells. (B and D) CFSE-labeled OT-I CD8+ T cells (3 × 106) were i.v. transferred into mice s.c. injected with 5 × 105 B16-OVA cells, together with s.c. injection of Th1 or Th9 cells at the same site of tumor cell inoculation on the same day. All mice (n = 3/group) were sacrificed 3 days later, and OT-I cells from TDLNs were analyzed by FACS after restimulation. (A and B) Upper panels show the frequency (%) of IFN-γ–producing CFSElo (proliferated) OT-I cells; lower panels show total CFSE+ OT-I cells and total CFSElo IFN-γ–producing OT-I cells recovered from TDLNs. (C and D) Histograms show granzyme B production by CFSE+ OT-I cells; graphs show mean fluorescence intensity of granzyme B expression by CFSE+ OT-I cells recovered from TDLNs. P values are shown as indicated.
Th9 cells help CD8+ CTLs in a Ccr6-dependent manner. IL-9, Th9 cells, or Th9 cell–derived supernatants did not have the function to help CD8+ CTL activation in vitro (data not shown), suggesting that Th9 cells indirectly provide help to induce CD8+ CTL response in vivo. The potential mediator of this help may be derived from DC populations, because we observed a large number of tumor tissue–infiltrating DCs and migration of these DCs into TDLNs (Supplemental Figure 9) in Th9 cell–treated mice. In fact, CD8α+ DCs increased more than 3-fold in lung tissues as early as 4 days after Th9 treatment, while there was only a slight increase in CD11b+ DCs (Supplemental Figure 10). As DCs are known to be predominantly responsible for the activation of CTLs in vivo, we hypothesized that Th9 cells may improve DC delivery of tumor antigens to TDLNs to prime and induce tumor-specific CD8+ CTL responses. We used mice bearing GFP-coexpressing B16-OVA tumor cells, which enabled us to identify the delivery of tumor materials to LLNs by GFP+ DCs. On day 3 after Th1 or Th9 cell transfer, we detected significantly higher percentages of both GFP+CD8α+ DCs and GFP+CD11b+ DCs in the LLNs of mice treated with Th9 cells as compared with PBS-treated mice, and Th1 cell treatment only slightly increased these cells (Figure 7A). Moreover, the absolute numbers of GFP+ DCs, especially GFP+CD8α+ DCs, were highly increased in the LLNs of mice treated with Th9 cells (Figure 7B).
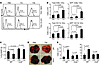 Figure 7
Figure 7Th9 cells regulate DC function in a Ccr6-dependent manner. (A) C57BL/6 mice (n = 3/group) were i.v. challenged with 1 × 105 B16-OVA-GFP tumor cells and, on the same day, were i.v. transferred with 3 × 106 Th1 or Th9 cells. LLNs were tested using FACS 3 days later for DC populations. Histograms show the percentage of GFP+ DCs gated on CD11c+CD8α+ DCs and CD11c+CD11b+ DCs. (B) Number of total and GFP+ cells for each DC population was calculated from A. Representative results from one of two performed experiments are shown. (C) PBS or 3 × 106 Th9 cells were transferred to mice (wild-type and Ccr6–/– mice; n =4/group) bearing 5-day established pulmonary B16-OVA melanoma. Shown are the lung weights on day 19 after challenge. (D) Representative images of the lungs of mice receiving different treatments. (E) LLN cells from C were analyzed for DC populations using FACS. The numbers of total CD8α+ DCs and CD11b+ DCs from mice (n = 4/group) are shown. Unless otherwise indicated, P values are shown as indicated.
To elucidate the mechanism underlying Th9 cell–regulated infiltration of DCs in the lung and subsequent migration to the LLNs, we hypothesized that Th9 cell–induced Ccl20 in the tumor tissues may be responsible for the inflammation in the lung. Since we and others (14) observed that both CD8α+ DCs and CD11b+ DCs expressed surface Ccr6 (Supplemental Figure 8), we then treated B16-OVA–bearing Ccr6–/– mice with adoptive transfer of Th9 cells. On day 19 after tumor challenge, we observed significantly larger tumor burdens in the Ccr6–/– mice compared with wild-type mice receiving PBS treatment (Figure 7, C and D), although the numbers of tumor foci were not significantly different between Ccr6–/– and wild-type mice receiving PBS treatment (Supplemental Figure 11). Furthermore, Th9 cell treatment failed to exert antitumor activity in the Ccr6–/– mice (Figure 7, C and D). This was associated with a decreased infiltration of both CD8α+ DCs and CD11b+ DCs in the LLNs (Figure 7E). Compared with the wild-type mice receiving Th9 cells, Th9 cell–treated Ccr6–/– mice showed significantly decreased numbers of infiltration, in the lung tumor tissue, of activated CD8+ and CD4+ T cells and CD8α+ DCs, and slightly decreased CD11b+ DCs and granulocytes (Supplemental Figure 12). These results together demonstrated that Th9 cells provide help to promote CD8+ CTL activation through Ccl20/Ccr6-dependent recruitment of DCs to load tumor antigens in the tumor tissues and through subsequent delivery of antigens and priming of CD8+ T cells in the TDLNs.
-
Discussion
IL-9 is a T cell growth factor, and dysregulated IL-9 signaling in lymphoid cells may lead to autonomous cell growth and malignant transformation (33). Accumulating evidence also showed that IL-9 and Th9 have pleiotropic functions on the immune system and play different roles in different situations, both positively and negatively regulating the immune responses (34). However, little is known about the role of IL-9 in tumor development, especially in tumor immunity. In this study, we observed an accelerated tumor growth of pulmonary melanoma in IL-9–neutralized mice, indicating a protective role for endogenous IL-9. More importantly, transfer of tumor-specific Th9 cells induced potent antitumor immune responses in both prophylactic and therapeutic tumor models by promoting strong antitumor CD8+ CTL response in vivo.
In line with our observation, a recent study showed that blockade of endogenous IL-9 accelerated the growth of s.c.-implanted melanoma (35). Those authors hypothesized that mast cells were responsible for IL-9–mediated antitumor activity when exogenous IL-9 was administrated in vivo, although how mast cells modulate the antitumor activity in response to IL-9 has not been determined. However, we found that the number of tumor tissue–infiltrating mast cells was extremely low and there were no detectable activated mast cells within the tumor tissues of both PBS and Th9 cell–treated mice.
IL-9 and Th9 induce expression of Ccl20 and Ccl11 (36, 37), which partially contributes to the tissue infiltration of leukocytes in autoimmune diseases and allergic inflammation. In IL-9–neutralized mice, we found sharply decreased Ccl20 expression in the lung tumor tissues, which was associated with impeded infiltration of Ccr6+ leukocytes as well as activated CD8+ effector T cells, thus rendering these mice more susceptible to tumor development. On the contrary, Th9 cell–transferred mice developed an inflammatory response in the lung tumor tissues and mounted strong antitumor CTL responses in a Ccl20/Ccr6-dependent manner. Our findings thus identify that the pro-inflammatory chemokine Ccl20 was crucial for Th9- and IL-9–induced antitumor immune responses in vivo.
As IL-10–producing cells, Th9 cells do not suppress T cell proliferation in vitro or inhibit tissue inflammation in vivo (22). The IL-9–secreting phenotype of Th9 has been reported to be unstable in some autoimmune disease models, and Th9 cells recovered from inflamed site or LNs produced predominantly IFN-γ (22, 32, 38). In our pulmonary melanoma model, however, Th9 cells maintained their IL-9 and IL-10 production in LLNs without switch to produce IFN-γ or IL-17. Interestingly, there were relatively high levels of IFN-γ production in the LLNs of mice receiving Th9-cell transfer, indicating that Th9 cell transfer induced activation of host immune effector cells. More importantly, we observed IFN-γ and IL-17 production in the lung of Th9 cell–transferred mice, whereas these cytokines were absent in the lung of Th1 cell–transferred mice, examined on day 4 after transfer, even though Th1 cells produced high levels of IFN-γ in the LLNs (Figure 4). These results suggest that Th9 cells not only induce effector cell activation in TDLNs, but also recruit them into the tumor sites to exert their tumor killing functions. On the other hand, Th1 cells almost only homed to LNs, and both Th1 cells and host effector T cells largely failed to migrate to the tumor sites.
Th1 cells have traditionally been regarded as most efficient in tumor rejection among the subsets of Th cells (4, 5). In the current study, Th9 cells, without any direct cytotoxic effect on tumor cells in vitro, provide better protection than Th1 cells in tumor-bearing mice. The antitumor activity of Th9 cells in vivo is probably due to the enhanced inflammatory responses induced in the tumor tissues and subsequently upregulated antigen presentation mediated by DCs. The suppressive milieu created by tumor cells usually inhibits the infiltration of leukocytes (39–41), especially DCs and effector T cells, thereby hindering tumor immunosurveillance. Th9 cell transfer resulted in substantially increased DC recruitment to the lung tumor tissues, where they loaded tumor antigens, migrated into TDLNs, and primed tumor-specific CD8+ T cells. The migration of tumor antigen–loaded DCs into TDLNs in Th9 cell–treated mice indicates that these DCs were programmed toward an immunogenic rather than tolerogenic status (42). Such migration resulted in a significant increase of tumor-specific CD8+ CTL activation in TDLNs, with sustained IFN-γ and granzyme B expression and subsequent homing of the CD8+CTLs to the tumor tissues and killing of tumor cells. The concept for revitalizing the capacity of immunogenic DCs to stimulate CTLs is widely accepted to be a critical step toward enhancing antitumor immunity (43).
It has become clear that development and/or maintenance of memory CD8+ T cells depend on CD4+ Th cells, although the mechanisms of help for CTL response is still debated (44). Our study, for the first time, demonstrates that Th9 cells provide help to the generation of effector and/or memory CD8+ CTLs in antitumor immunity. In contrast to Th1 cells, Th9 cells recruit DCs — especially CD8α+ DCs — for cross-presentation of tumor antigens to CD8+ T cells in a Ccl20/Ccr6-dependent manner. CD8α+ DCs are specialized for cross-presentation of antigen by MHC class I molecules to CD8+ T cells and are reported to be crucial for antiviral and antitumor CTL responses (14, 45, 46). On the contrary, CD11b+CD8α– DCs are the major subset of DCs in the lung tumor tissues of PBS- and Th1 cell–treated mice, and have been considered to be tolerogenic and promote Th2 responses (47, 48). Ccr6 deficiency selectively impaired the recruitment of CD8α+ DCs, abrogated the infiltration of activated CD8+ CTLs, and compromised the antitumor responses mediated by Th9 cells.
We therefore propose the following model, based on the findings of this study: (a) tumor-infiltrating Th9 cells induce lung tumor tissues, especially epithelial cells, to express Ccl20; (b) Ccl20 provokes inflammation in tumor tissues and recruits leukocytes, especially CD8α+ DCs, into tumor sites; (c) DCs load antigens in tumor tissues and migrate into TDLNs to prime and activate host effector cells; (d) activated host effector cells, especially CD8+ CTLs, migrate into tumor sites via Ccl20 chemoattraction and kill tumor cells. Our study thus suggests a distinct role for tumor-specific Th9 cells in provoking CD8+ CTL-mediated antitumor immunity, which offers a novel approach to enhancing CD8+ CTL-based cancer immunotherapy.
-
Methods
Mice and cell lines. C57BL/6 mice were purchased from the National Cancer Institute, and OT-II (C57BL/6-Tg[TcraTcrb]425Cbn/J), OT-I (C57BL/6-Tg[TcraTcrb]1100Mjb/J), Ifng–/– (B6.129S7-IFN-γtm1Ts/J), and Ccr6–/– (B6.129P2-Ccr6tm1Dgen/J) mice were purchased from The Jackson Laboratory. All mice were 6 to 8 weeks old at the beginning of each experiment. Wild-type B16 or B16-OVA melanoma cell lines were cultured in Iscove’s modified Dulbecco’s media (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Thermo Scientific), 100 U/ml penicillin-streptomycin, and 2 mM L-glutamine (both from Invitrogen).
In vitro Th9 and Th1 cell differentiation. Naive CD4+CD62L+ T cells purified from spleens of OT-II mice were differentiated into Th0, Th1, or Th9 cells according to established methods (32). OVA-specific naive CD4+ T cells were cultured with irradiated splenic APCs from C57BL/6 mice in the presence of OVA323–339 peptide (5 μg/ml; Abgent) with Th9-polarized medium supplemented with IL-4 (10 ng/ml; R&D Systems), TGF-β (1 ng/ml; R&D Systems), and anti–IFN-γ mAbs (10 μg/ml; eBioscience) or with Th1-polarized medium supplemented with IL-2 (30 ng/ml; R&D Systems), IL-12 (4 ng/ml; R&D Systems), and anti–IL-4 mAbs (10 μg/ml; eBioscience). Th0 cells were plated in the absence of exogenous cytokines and mAbs. After 3 days of culture, differentiated Th1 or Th9 cells were depleted of dead cells and used in animal studies.
In some experiments, cells were restimulated for 5 hours with OVA peptide in the presence of GolgiPlug (BD Biosciences) before ICS using a Cytofix/Cytoperm kit (BD Biosciences) or Foxp3 staining kit (eBioscience). Cytokine levels in supernatants were measured using ELISA kits (Peprotech).
Induction of B16 lung melanoma. Mice were injected i.v. with 1 × 105 B16 or B16-OVA cells. For adoptive transfer experiments, mice were injected i.v. on the same day with 3 × 106 OVA-specific Th1 or Th9 cells in the prevention model or 5 days after tumor injection in the therapeutic model. At day 18 or 19 after tumor injection, mice were sacrificed for enumeration of metastatic lung foci. All lung lobes were evaluated under a tissue microscope. In the experiments in which α–IL-9 (clone D9302C12; BD Biosciences), α–IL-10 (clone JES5-2A5; BioXcell), or anti-CD8 (clone 2.43; BioXcell) was used, 100 μg/mouse was injected i.p. every 3 days.
Tumor induction s.c. Mice were injected s.c. in the front of the abdomen with 2 × 105 or 5 × 105 B16-OVA, and on the same day, some mice received i.v. injection of 3 × 106 OVA-specific Th1 or Th9 cells or s.c. injection of 2 × 106 OVA-specific Th1 or Th9 cells at the same site of tumor cell injection.
Fractionation of the lung. Lungs were digested with 1 mg/ml of collagenase D (Roche Applied Science) for 30 minutes at 37°C, and with 0.01 mM EDTA (Sigma-Aldrich) for 5 minutes, to prevent aggregates. The cells were collected using Ficoll-Paque (GE Healthcare) density gradient centrifugation. The middle section of the gradient was enriched for leukocytes, counted, and used for analysis. In some experiments, lung leukocytes were purified through sorting of CD45+ cells.
Lung epithelial cells were isolated as described previously (49), with some modifications. Lungs were inflated with dispase (Roche Applied Science) to collapse, and then agitated in dispase at room temperature for 30 minutes. Lungs were then minced and digested with collagenase and DNase I (Sigma-Aldrich). Cell suspension was filtered and incubated on precoated dishes containing α-CD45 and α-CD32 antibodies (eBioscience) for 2 hours. Nonadherent epithelial cells were panned from the antibody plates and used for analyses.
Flow cytometry. FITC-, PE-, APC-, or PerCP-conjugated mAbs against CD45, CD4, CD8, CD11c, CD11b, CD44, Gr1, F4/80, IL-9, IFN-γ, IL-4, IL-10, Foxp3, and IL-17 (all from eBioscience) were used for staining after Fc blocking, and analyzed using a FACSCalibur flow cytometer. Kb tetramer carrying the OVA257–264 was purchased from Beckman Coulter.
CFSE labeling and transfer. Th1, Th9, or OT-I CD8+ T cells were incubated for 5 minutes at 37°C with 1 μM CFSE in PBS, and then washed extensively. OVA-specific CFSE-labeled Th1 or Th9 cells (3 × 106) were transferred i.v. into mice bearing 5-day pulmonary B16-OVA melanoma. Mice were sacrificed 4 days later, and CFSE+ Th1 or Th9 cells and CFSE– endogenous cells were sorted from LLNs with a flow cytometer. CFSE+ Th1 or Th9 cells were maintained with 10 U/ml IL-2, and CFSE– cells were restimulated with 5 μg/ml OVA323–339 and OVA257–264 peptides for 36 hours. In some experiments, 3 × 106 OVA-specific Th1 or Th9 cells were i.v. transferred into mice bearing 5-day pulmonary B16-OVA melanoma. Mice were sacrificed 4 days later, and cells from leukocyte fraction of lungs or LLNs were restimulated with 5 μg/ml OVA323–339 and OVA257–264 peptides for 36 hours. Supernatants were collected, and cytokine levels were measured with ELISA kits (Peprotech).
In some experiments, 3 × 106 CFSE-labeled OT-I CD8+ T cells were transferred i.v. into mice bearing 5-day pulmonary B16-OVA melanomas, which were also i.v. transferred with 3 × 106 Th1 or Th9 cells. In other experiments, mice were transferred i.v. with CFSE-labeled OT-I CD8+ T cells (3 × 106) and injected s.c. with 5 × 105 B16-OVA cells, together with s.c. injection of Th1 or Th9 cells at the same site of tumor cell inoculation on the same day. Mice were sacrificed 3 days later, and cells from the leukocyte fraction of lung or LLNs were restimulated with 5 μg/ml OVA257–264 peptide with GolgiPlug (BD Biosciences) for 6 hours. ICS staining for IFN-γ and granzyme B was performed using Cytofix/Cytoperm kit (BD Biosciences).
Histology. Formalin-fixed, paraffin-embedded lung sections were incubated with α-Ccl20 antibodies (Abcam) and then incubated with FITC-conjugated secondary antibodies (Invitrogen). Immunofluorescence was examined with a fluorescence microscope. Mast cells were visualized using toluidine blue staining on formalin-fixed, paraffin-embedded lung sections.
Real-time PCR. Total RNA was extracted from lung tumor tissues by using an RNeasy Mini kit (Qiagen) according to the manufacturer’s instructions. The expression of Il9r, Ccl20, Ccl2, Ccl7, Ccr6, Ccr2, and Cxcl1 was analyzed using specific primers and analyzed with SYBR Green real-time PCR (Applied Biosystems). Expression was normalized to the expression of the housekeeping gene β-actin.
Statistics. For statistical analyses, a 2-tailed Student’s t test was used. A P value less than 0.05 was considered statistically significant. Results are presented as means ± SD.
Study approval. Animal studies were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
-
Acknowledgments
This work was supported by funds from the Center for Targeted Therapy at The University of Texas MD Anderson Cancer Center, grants from the National Cancer Institute (R01CA138402, R01CA138398, R01CA163881, and P50CA142509), the Leukemia and Lymphoma Society, the Multiple Myeloma Research Foundation, and the Commonwealth Foundation for Cancer Research.
Address correspondence to: Qing Yi, MD Anderson Cancer Center, Department of Lymphoma and Myeloma, 1515 Holcombe Boulevard, Unit 903, Houston, Texas 77030, USA. Phone: 713.563.9065; Fax: 713.563.9241; E-mail: [email protected].
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2012;122(11):4160–4171. doi:10.1172/JCI65459.
See the related article at Amazing IL-9: revealing a new function for an “old” cytokine.
-
References
- Petermann F, Korn T. Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett. 2011;585(23):3747–3757.
- Gerloni M, Zanetti M. CD4 T cells in tumor immunity. Springer Semin Immunopathol. 2005;27(1):37–48.
- Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–728.
- Nishimura T, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl):S52–S61.View this article via: PubMed Google Scholar
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6(11):836–848.
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–2368.
- Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23(11):3043–3049.
- Wilke CM, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32(5):643–649.
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464.
- Chen X, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69(3):348–354.
- Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373.
- Muranski P, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35(6):972–985.
- Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated CD8(+) T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother. 2011;60(10):1473–1484.
- Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798.
- Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci. 2012;1247:56–68.
- Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10(10):683–687.
- Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127(4):450–458.
- Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189(5):419–435.
- Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186(6):3283–3288.
- Schmitt E, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153(9):3989–3996.View this article via: PubMed Google Scholar
- Veldhoen M, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9(12):1341–1346.
- Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9(12):1347–1355.
- Perumal NB, Kaplan MH. Regulating Il9 transcription in T helper cells. Trends Immunol. 2011;32(4):146–150.
- Li H, Nourbakhsh B, Cullimore M, Zhang GX, Rostami A. IL-9 is important for T-cell activation and differentiation in autoimmune inflammation of the central nervous system. Eur J Immunol. 2011;41(8):2197–2206.
- Nowak EC, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206(8):1653–1660.
- Elyaman W, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(31):12885–12890.
- Smith SE, Hoelzinger DB, Dominguez AL, Van Snick J, Lustgarten J. Signals through 4-1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol Immunother. 2011;60(12):1775–1787.
- Eller K, et al. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol. 2011;186(1):83–91.
- Stephens GL, et al. IL-9 is a Th17-derived cytokine that limits pathogenic activity in organ-specific autoimmune disease. Eur J Immunol. 2011;41(4):952–962.
- Wu B, et al. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin Immunol. 2008;126(2):202–210.
- Yamazaki T, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–8401.View this article via: PubMed Google Scholar
- Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183(11):7169–7177.
- Knoops L, Renauld JC. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors. 2004;22(4):207–215.
- Li H, Rostami A. IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neuroimmune Pharmacol. 2010;5(2):198–209.
- Purwar R, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells [published online ahead of print July 8, 2012]. Nat Med.
doi:
10.1038/nm.2856
.
View this article via: PubMed Google Scholar
- Zhou Y, et al. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J Immunol. 2011;186(7):4415–4421.
- Yamasaki A, Saleh A, Koussih L, Muro S, Halayko AJ, Gounni AS. IL-9 induces CCL11 expression via STAT3 signalling in human airway smooth muscle cells. PLoS One. 2010;5(2):e9178.
- Tan C, et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol. 2010;185(11):6795–6801.
- Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13(18 Pt 1):5256–5261.
- Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200(6):771–782.
- Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205(9):2125–2138.
- Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205(4):825–839.
- Sheng KC, Wright MD, Apostolopoulos V. Inflammatory mediators hold the key to dendritic cell suppression and tumor progression. Curr Med Chem. 2011;18(36):5507–5518.
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4(8):595–602.View this article via: PubMed Google Scholar
- Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–111.
- GeurtsvanKessel CH, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205(7):1621–1634.
- Kriegel MA, Rathinam C, Flavell RA. Pancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c+ CD11b+ dendritic cells. Proc Natl Acad Sci U S A. 2012;109(9):3457–3462.
- Maldonado-Lopez R, et al. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189(3):587–592.
- Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L256–L264.View this article via: PubMed Google Scholar
-
Version history
- Version 1 (October 15, 2012): No description
- Version 2 (November 1, 2012): No description



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)







