Advertisement
Review Series Free access | 10.1172/JCI31446
Principles of adoptive T cell cancer therapy
Carl H. June
Abramson Family Cancer Research Institute and Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Address correspondence to: Carl H. June, University of Pennsylvania, 421 Curie Blvd., Philadelphia, Pennsylvania 19104-6160, USA. Phone: (215) 573-5745; Fax: (215) 573-8590; E-mail: [email protected].
Find articles by June, C. in: JCI | PubMed | Google Scholar
Published May 1, 2007 - More info
J Clin Invest. 2007;117(5):1204–1212. https://doi.org/10.1172/JCI31446.
© 2007 The American Society for Clinical Investigation
-
Abstract
The transfusion of T cells, also called adoptive T cell therapy, is an effective treatment for viral infections and has induced regression of cancer in early-stage clinical trials. However, recent advances in cellular immunology and tumor biology are guiding new approaches to adoptive T cell therapy. For example, use of engineered T cells is being tested as a strategy to improve the functions of effector and memory T cells, and manipulation of the host to overcome immunotoxic effects in the tumor microenvironment has led to promising results in early-stage clinical trials. Challenges that face the field and must be addressed before adoptive T cell therapy can be translated into routine clinical practice are discussed.
-
Introduction
Adoptive T cell therapy for cancer is a form of transfusion therapy consisting of the infusion of various mature T cell subsets with the goal of eliminating a tumor and preventing its recurrence. Allogeneic and autologous sources of T cells derived from several anatomic sites have been tested. Indeed, in the 1970s, Chester Southam and colleagues demonstrated that subcutaneous growth of human tumor autografts to patients bearing advanced cancers was inhibited by the cotransfer of autologous leukocytes in about half of the patients (1). This finding suggested that lymphocytes with a specific inhibitory effect on the implantation and growth of cancer cells were present in many patients and could be mined as potential candidates for adoptive immunotherapy. The primary advantage of using CD8+ T cells for adoptive therapy, as opposed to other cytolytic cells, such as NK cells, is their ability to specifically target tumor cells through the recognition of differentially expressed tumor proteins presented on the cell surface. Using T cells for adoptive therapy is also attractive due to the long clonal life span of T cells (2, 3), which allows both therapeutic and immunoprophylactic scenarios to be envisioned. In addition, T cells are well suited for genetic manipulation, which has enabled the evaluation of genetically enhanced or retargeted T cells in pilot clinical trials for cancer as well as other diseases. The intent of this Review on adoptive T cell therapy for cancer is to address current issues facing this field, with a focus on translational issues relevant to human immunotherapy. For earlier references, the reader is referred to previous reviews on adoptive transfer (4–6).
Adoptive T cell therapy depends on the ability to optimally select or genetically engineer cells with targeted antigen specificity and then induce the cells to proliferate while preserving their effector function and engraftment and homing abilities. Unfortunately, many clinical trials have been carried out with adoptively transferred cells that were propagated in what are now understood to be suboptimal conditions that impair the essential functions of T cells. In addition, until recently, many trials were carried out before the complexity of T cell biology was understood. Some important issues that were previously not understood but might have affected the outcome of many of the early trials are discussed in this Review. These issues include the important differences in T cell biology between rodents and humans that have emerged in recent years (7), the observation that T cell populations are heterogeneous and comprise memory cells, effector cells, and Tregs, and the process of immunosenescence. The implications of these findings need to be incorporated into the translation of therapeutic approaches from animal models to the clinic. In addition, although the principles of T cell biology should guide the ex vivo culture process, in some instances it is necessary to compromise in order to achieve a cell manufacturing approach that is robust enough to support large scale trials and is compliant with FDA guidelines and other regulations (8).
-
Use of the laboratory mouse for adoptive T cell therapy: curse or blessing?
Mouse syngeneic tumor models have been essential for the identification and preclinical optimization of many tumor therapies. However, so far, no mouse tumor model has been a good predictor of a successful human response to immunotherapy. For example, it is instructive to note that in mice, treatment with cytotoxic T lymphocyte–associated antigen 4–specific (CTLA4-specific) antibody induces antitumor responses and is well tolerated (9) whereas in humans, antitumor responses can be accompanied by life-threatening colitis and hypophysitis (10, 11). Over recent years, substantial limitations in some mouse models have emerged that have important implications for adoptive immunotherapy. In particular, the critical demonstration that many mouse tumor antigens are not representative of human tumor antigens (12) has led to improved strategies to generate adoptively transferred human T cells with appropriate tumor specificity. With the exception of virally induced cancers, which account for about 10% of human tumors, clinical trials target self antigens, a technically challenging task since tolerance and TCR repertoire limitations can restrict the quality of the T cell response (13). Furthermore, the seminal discovery of telomerase (14) and the developing field of telomere biology identified fundamental differences in mechanisms of immunosenescence between mice and humans (15–17) that have important implications for adoptive T cell transfer therapeutics. These issues of immunosenescence and limitations of repertoire are discussed in more detail below.
CD28, a membrane glycoprotein that is a costimulatory receptor for TCR-mediated T cell activation, is the main costimulatory molecule for T cell activation in mice and humans (18). In humans, but not mice, there is an accumulation of CD8+CD28– T cells in the peripheral blood with age and with increasing length of ex vivo culture (19). CD8+CD28– T cells are often oligoclonal in nature and do not proliferate well in response to antigenic stimulation (20). Because almost all T cells in the peripheral blood of newborn humans express CD28 and because the accumulation of CD28– T cells is a gradual process with age, it has been suggested that CD8+CD28– T cells are derived from CD8+CD28+ T cells (21). The mechanism of CD28 downregulation in human T cells is not fully understood but involves decreased CD28 transcription (22). To the extent that loss of CD28 expression with prolonged in vitro culture models the aging human immune system (23), this suggests the importance of developing strategies to preserve CD28 expression on adoptively transferred human T cells. CD28-specific agonist antibodies have been useful for ex vivo stimulation of human T cells for adoptive therapy (24). However, for reasons that still require clarification, the in vivo administration of CD28-specific agonist antibodies, although well tolerated in rodents, is highly toxic in humans (25). Together, the above examples illustrate the need for improved adoptive T cell transfer models. Recent advances in the development of humanized mouse models might circumvent these issues (26). Furthermore, despite these downsides, syngeneic mouse models have been valuable in determining the optimal lymphocyte subsets and culture conditions for adoptive T cell therapy (5).
-
Striving for optimal T cell function
T cells exist in several distinct stages of differentiation. Naive CD4+ and CD8+ T cells undergo unique developmental programs after antigen activation, resulting in the generation of effector memory and long-lived central memory T cells, TEM cells and TCM cells, respectively. Three models by which memory CD8+ T cells can be generated have been proposed (Figure 1). In the linear differentiation model, an autonomous antigen-triggered differentiation process consisting of conversion from naive to effector to TEM cell occurs, followed by the appearance of TCM cells after antigen clearance through a process of dedifferentiation (27). The signal strength model proposes that naive T cells progress through hierarchical thresholds for proliferation and differentiation as the strength and duration of the interaction with APCs is increased (28). T cells receiving the weakest signals do not survive whereas high-intensity signaling causes the development of terminally differentiated effector T cells that cannot survive into the memory phase. The TCM cells, being the least differentiated of the antigen-stimulated T cells, retain the developmental options of naive T cells, including their capacity for marked clonal expansion. The stem cell model proposes that the cells within the TCM cell compartment are self renewing and serve as a source of effector T cells (29). Although the details and mechanisms of differentiation remain to be clarified, it is clear that the various T cell memory subsets have specialized roles and that not all the subsets would be efficacious in the setting of adoptive T cell therapy for the treatment of cancer.
 Figure 1
Figure 1Models of CD8+ T cell differentiation to distinct memory cell subsets. (A) In the linear differentiation model, an autonomous antigen-triggered differentiation process consisting of conversion from naive to effector to TEM cell occurs, followed by the appearance of TCM cells after antigen clearance through a process of dedifferentiation (27). (B) The signal strength model proposes that naive T cells progress through hierarchical thresholds for proliferation and differentiation as the strength and duration of the interaction with APCs is increased (28, 30). T cells receiving the weakest signals do not survive, whereas high-intensity signaling causes the development of terminally differentiated effector T cells that cannot survive into the memory phase. The TCM cells, being the least differentiated of the antigen-stimulated T cells, retain the developmental options of naive T cells, including their capacity for marked clonal expansion. (C) The memory stem cell model proposes that the cells within the TCM cell compartment are self renewing and serve as a source of effector T cells (29, 43).
At present, naive T cells are not thought to be useful for adoptive transfer as they are unable to kill tumor cells. The demonstration by Sallusto and colleagues (30) that TCM and TEM cell subsets have discrete trafficking and functional properties has the potential to fundamentally alter approaches to adoptive T cell therapy. In retrospect, previous clinical trials have primarily tested adoptively transferred populations of CD8+ TEM cells (31). This approach was taken because available tissue culture technologies resulted in rapid differentiation of T cells to late-stage effector cells; late-stage TEM cells express CD57 and have poor replicative capacity (32, 33). In vitro, TEM cells are superior to TCM cells at tumor cytotoxicity. However, in vivo, TCM cells exhibit superior therapeutic effects when compared with TEM cells on a per cell basis (31, 34). Therefore, in principle, adoptive T cell transfer strategies are attractive for the ability to generate long-lived populations of TCM cells capable of immune surveillance as well as tumor eradication, which is why efforts to test TCM cells in clinical trials are currently of high priority.
Factors determining the optimal CD8+ T cells for adoptive transfer. The cellular basis of immunologic memory has been one of the central issues of immunology for more than half a century. Many studies in mice indicate that true memory with long-lived T cells is only established in the absence of persistent antigen (35, 36). This raises questions for tumor immunologists as to whether central memory can be established in tumor-bearing patients and, if so, how this establishment would occur. Relevant to this issue is that in patients at risk of developing EBV lymphoma, adoptively transferred gene-marked CTLs persist for years (37), demonstrating that in principle it is possible to establish central memory in cancer patients by adoptive T cell transfer. Therefore, to understand approaches to generating persistent immunity in patients in whom tumor antigens are unlikely to be completely eliminated, it might be more instructive to study the human immune response in patients with chronic persistent viral and parasitic infections (38, 39). A major issue of relevance is the differentiation pathway leading to TCM cells (Figure 1). Some studies indicate that naive T cells first differentiate into effector T cells, a proportion of which then differentiate into TCM cells (40). In contrast to this linear differentiation model, other studies suggest that parallel differentiation occurs, with naive T cells directly differentiating into TCM and effector cells simultaneously through asymmetric division (41, 42). Resolution of the above issues is important so that culture systems can be devised to optimally derive populations of TEM and TCM cells.
In the mouse, a memory stem cell subset of CD8+ T cells has recently been described (43). These cells were identified based on alloreactivity and had extensive replicative potential in vivo but maintained the naive CD44–CD62L+ T cell phenotype. Intriguingly, unlike naive T cells, these newly identified CD8+ T cells expressed high levels of stem cell antigen 1 (SCA1), a glycosylphosphatidylinositol-linked molecule found on self-renewing cells from various tissues. In humans, the existence of TCM cells with stem cell–like qualities has been proposed (28), but these cells have not been clearly identified, preventing the design of adoptive immunotherapy strategies to test this putative subset of cells. Assuming that the existence of the mouse memory stem cell is confirmed, it will be important to determine whether a similar stem cell subset exists in human T cells.
In humans, adoptive T cell therapy approaches to date have used peripheral blood, tumors, malignant effusions, and draining lymph nodes as the anatomic sources of input T cells for adoptive transfer (for examples, see refs. 44–47). Given the recent demonstration that the bone marrow is a major reservoir of self-reactive T cells (48), it is important to determine whether improved antitumor effects are observed when bone marrow–resident T cells are used for adoptive transfer. The bone marrow of breast cancer patients has been shown to contain CD8+ T cells specific for peptide epitopes from the tumor antigens MUC1 and HER2/neu (49), and adoptive transfer of bone marrow–derived human CD8+ memory T cells mediates antitumor activity in mice bearing tumor xenografts (50). Furthermore, bone marrow from patients with either pancreatic cancer or myeloma has also been shown to be enriched for tumor-reactive CD8+ T cells (51–54).
A role for CD4+ T cells in adoptive T cell transfer. Many studies show that the generation and/or maintenance of CD8+ T cell memory requires CD4+ T cell help (55) and that immunity specific for tumors lacking expression of MHC class II molecules is enhanced with CD4+ T cell help (56). It is counterintuitive that CD4+ T cells enhance antitumor effects in hosts bearing tumors that lack MHC class II. However, adoptively transferred CD4+ T cells have the potential to augment tumor immunity by several mechanisms that might enhance the survival and function of CD8+ T cells, including the secretion of essential cytokines such as IL-2 and IL-21 (57) and the expression of CD40L (58). Besides their intimate involvement in priming tumor-specific CTLs, CD4+ cells participate in additional effector functions. Evidence indicates that other cytokines produced by CD4+ T cells can recruit and activate macrophages and eosinophils that, in turn, mediate antitumor effects (59). Clinical adoptive transfer studies also show that the persistence of adoptively transferred cytotoxic CD8+ effector T cells is enhanced with the concomitant administration of IL-2 (60) or CD4+ T cells (61). Recent studies in patients with myeloma show that the adoptive transfer of mixed populations of pathogen-specific CD4+ and CD8+ T cells promoted the establishment of immunity with a robust central memory component (62). However, it is not yet known if this approach enhances the establishment of immunity to self antigens in cancer patients.
The common γ chain (γc) is a shared receptor component for the IL-2, IL-4, IL-7, IL-9, and IL-15 receptors. In the mouse, IL-15 is not produced by T cells whereas human CD4+ memory T cells are reported to constitutively produce and proliferate in response to the cytokine IL-15 (63), suggesting different roles for IL-15 in mouse and human CD4+ memory T cell homeostasis (64). Genetic analysis of patients with a γc deficiency indicate that the human is absolutely dependent on cytokines that signal through γc for T cell development, as T cells are absent in these individuals, whereas in γc-deficient mice the spleens of older mice have nearly normal numbers of CD4+ T cells (65), indicating that γc-independent signals can support CD4+ T cell development in mice but not humans. In mice, adoptively transferred CD8+ T cells cultured in IL-15 show more tumor cytotoxicity than those cultured in IL-2 (31), so the use of IL-15 for adoptive T cell therapy in humans holds substantial promise of augmenting T cell numbers and effector functions, although species differences can be expected.
At present, one of the most important issues facing the field is the complexity of CD4+ lineage T cells. Until recently, Th cells were separated into two different subsets named Th1 and Th2cells, based on the pattern of cytokines that they produce when stimulated. However, several types of CD4+ Tregs have now been described in humans (66), and it is probably important to remove Tregs from adoptively transferred T cell populations because they suppress antitumor immunity (67). Since Tregs are often enriched in tumor-infiltrating lymphocytes and the peripheral blood in cancer patients (68), it is possible that the outcomes of previous adoptive T cell therapy clinical trials were compromised because the adoptively transferred T cell populations inadvertently contained Tregs.
Of late, a fourth axis to the CD4+ T cell lineage was recognized, as a new IL-23–driven subset of IL-17–producing CD4+ T cells called Th17 has been described (69). Th17 cells are proinflammatory, but paradoxically they seem to have tumor immunosuppressive effects. An interesting finding is that tumors express IL-23, which might decrease immune surveillance of tumors by preventing Th1 cell–driven CD8+ T cell infiltration (70). Therefore, a newly recognized form of immune evasion is the diversion by tumors of immune responses from Th1- to Treg- and Th17-dominated CD4+ responses. However, as evidence from several groups indicates that IL-17–producing Th17 cells, rather than, as once was thought, IFN-γ–producing Th1 cells, represent the key effector cells in the induction of autoimmunity (71), it remains possible that adoptive transfer of Th17 cells might augment antitumor responses.
-
Developing optimal cell culture systems
The only forms of adoptive cellular therapy routinely employed in the practice of medicine are allogeneic bone marrow and peripheral blood stem cell transplantation (72). In this setting, donor leukocyte infusions mediate various potent antitumor effects (73). The adoptive transfer of activated donor (allogeneic) T cells shows promise of augmenting this effect (74, 75). Ex vivo culture approaches to altering the ratio of effector T cells to Tregs has the potential of decreasing the risk of graft-versus-host disease while preserving antitumor effects (76). However, for most patients, comorbidity and logistic issues related to the identification of suitable allogeneic donors mean that autologous approaches are preferred. Therefore, a central issue for the development of clinical adoptive T cell therapy strategies has been the development of culture systems in order to produce adequate numbers of effector T cells for autologous therapy.
Two basic approaches are being tested for clinical adoptive T cell therapy (Figure 2). The first approach is to isolate and activate in vitro antigen-specific T cells from peripheral blood or tumor specimens and then to use repetitive stimulation to clonally expand in vitro the antigen-specific T cells by various approaches. In the second approach, polyclonal ex vivo activation of the T cells is done based on three assumptions: first, tumor-specific T cells are present in the patient; second, the tumor-specific T cells are primed in the patient; and third, the in vivo function of the tumor-specific T cells in the patient is impaired. In the second approach, the cells are activated in a polyclonal fashion by various means in vitro and are then reinfused into the patient with the expectation that they will respond directly to the tumor or to tumor antigens presented by APCs. The first approach guarantees antigen specificity but is costly and labor intensive; the second approach is technically more rapid and feasible. In practical terms, only the second approach has been sufficiently robust to support randomized clinical trials (47, 62), and therefore, only this approach has the potential for regulatory approval. The rationale for the second approach has been substantially strengthened by the realization that many patients are already primed to their tumors (77, 78) and that the major challenge is improving the quality and quantity of the natural immune response (79). However, interest in both approaches has been reinforced by the realization that antigen-independent expansion of the transferred memory T cells can occur in vivo (80) under certain situations.
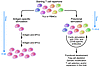 Figure 2
Figure 2General approaches for ex vivo T cell expansion. Input T cells are obtained from various anatomic sites, including peripheral blood and the tumor itself. Antigen-specific CTLs can be selected and expanded by repeated stimulation with antigen pulsed APCs or tumor cells. This process requires several rounds of stimulation (left). Alternatively, the starting T cell population can be numerically expanded by polyclonal stimulation by several methods to generate cells with enhanced effector function, to deplete Tregs, and to genetically modify T cells while maintaining the TCR repertoire of the input population (right). TIL, tumor-infiltrating lymphocyte.
Different ways to present antigen to T cells ex vivo. The most appropriate methods of ex vivo T cell culture mimic the physiologic processes whereby DCs generate a constellation of antigen-specific and costimulatory signals in the T cells. DCs are the most efficient APCs for the activation of naive T cells. However, although useful for therapeutic vaccination, due to practical considerations such as substantial manufacturing costs and the logistics of maintaining independent culture systems, DCs are not useful as APCs for large-scale adoptive T cell therapy trials. In addition, DCs have limited replicative potential; for ex vivo expansion of autologous T cells, it is desirable to have APCs with extensive replicative potential to facilitate both the scaling up of the process and multiple rounds of T cell stimulation. In addition, as was noted previously, since many patients are already primed to their tumors, other less potent forms of APCs might suffice to induce T cell activation. The best results to date have been with the rapid expansion method developed by Riddell and coworkers, which uses irradiated allogeneic peripheral blood mononuclear cells as APCs (also known as feeder cells) to expand CTLs for adoptive transfer (81). The main limitation of this approach is in scale-up because conforming to FDA-mandated requirements for the validation and qualification of allogeneic feeder cells can be tedious and expensive. Schultze and coworkers have shown that CD40-stimulated B cells, which have an extensive replicative potential, are an efficient means of propagating antigen-specific T cells (82). Therefore, although currently available tissue culture approaches have provided proof of concept for adoptive T cell therapy, a current priority is to develop alternative approaches that can support the large-scale trials required for FDA approval.
To generate antigen-specific T cells, cell lines and beads can be engineered to create artificial APCs and avoid the need to use autologous APCs for patient-specific cultures (reviewed in ref. 83). General approaches have been to produce artificial APCs either by coating beads with CD3-specific antibodies or peptide-MHC complexes or by transfecting cells that lack endogenous MHC molecules with MHC molecules and costimulatory molecules. Enhanced polyclonal T cell activation and proliferation result when cells are stimulated through the TCR and CD28 (84). In addition, CD28 stimulation maintains telomere length in human T cells, and this might improve engraftment and the persistence of the adoptively transferred T cells (85, 86). This culture system has been adapted for clinical use, and starting with an initial apheresis product, it is possible to generate the number of mature T cells found in an adult within two weeks of ex vivo culture (87, 88).
Magnetic beads coated with MHC class I molecules loaded with specific peptide have been used to elicit antigen-specific T cell propagation (89). Following isolation and expansion, cell populations generated using such beads specifically kill antigen-expressing target cells in vitro and display antiviral therapeutic effects in rodents (90). Others have used nonmagnetic microspheres coated with complexes of recombinant peptide–loaded MHC molecules to successfully generate CTLs ex vivo from naive precursors (91). Peptide-MHC tetramers presenting peptides from the melanoma tumor antigens MART1 (melanoma-associated antigen recognized by T cells 1) and gp100 (glycoprotein 100) have also been used to isolate high-avidity tumor-reactive CD8+ T cells from a heterogeneous population by flow cytometry. The tetramer-reactive cells have been cloned and retained their functional activity upon restimulation (92, 93). Sadelain and colleagues have engineered APCs that can be used to stimulate T cells of any patient expressing a specific HLA allele (94). Mouse fibroblasts were retrovirally transduced with a single HLA class I complex along with the human accessory molecules CD80 (also known as B7-1), CD54 (also known as ICAM-1), and CD58 (also known as LFA-3). These artificial APCs consistently elicited and expanded CTLs from patients of the appropriate haplotype specific for MART1 and gp100. We have also found that artificial APCs that express 4-1BB ligand efficiently expand human CD8+ TCM cells that have potent cytolytic function (89, 95, 96), and others have shown that CD83 expression on artificial APCs enhances the generation of CTLs (97).
Antigen concentration affects T cell stimulation ex vivo. It is critical that the correct concentration of antigenic peptide be used to pulse APCs. Alexander-Miller and colleagues showed that if high concentrations of peptide are used in vitro, only low-avidity T cells are propagated because the high-avidity T cells die by apoptosis (98), which suggests that submaximal concentrations of peptides might be required for in vitro induction of tumor-reactive CTLs. Furthermore, CTLs generated with peptide-pulsed APCs are often peptide reactive but not reactive with tumors that express the gene of interest due to low level expression or impaired antigen processing by the tumor cells. To circumvent this, Greenberg and colleagues have infected autologous APCs with recombinant vaccinia virus encoding the melanoma-associated antigen tyrosinase and have generated tyrosinase-specific and melanoma-reactive CTLs from the peripheral blood of 5 out of 8 patients with melanoma (99). In addition, tyrosinase-specific CD4+ T cell clones were isolated from 6 of the 8 patients by stimulation with the autologous engineered APCs, and all these clones were able to recognize autologous tumor cells. This approach and other similar approaches (100) increase the probability that the expanded T cells recognize peptides that are presented by tumors. In addition, because the APCs express full-length protein sequences, this strategy permits the isolation of T cells that recognize epitopes not previously defined, circumventing the problem of not having identified all tumor antigens and not knowing which peptides are most efficiently presented. Furthermore, this approach permits the isolation of CD4+ and CD8+ tumor-reactive T cells from the peripheral blood of patients with cancer, perhaps obviating the need to use tumor-infiltrating T cells as the input population in ex vivo T cell expansion protocols.
-
Telomeres: size does matter!
Telomeres have been shown to be involved in the control of cell proliferation, the regulation of cell senescence, and the unlimited proliferative capacity of malignant cells (14, 15). Human telomeres are composed of TTAGGG repeats at the chromosomal ends, and they function as tumor suppressors to protect chromosomes from degradation, fusion, and recombination (Figure 3).
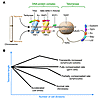 Figure 3
Figure 3Telomeres and telomerase function in T cell subsets. (A) In mammalian cells, telomeres are structures at the ends of all linear chromosomes that consist of hexanucleotide repeats [(TTAGGG)n] and several associated protein complexes. The two components of telomerase are illustrated — TERC and TERT. NBS, Nijmegen breakage syndrome; MRE, meiotic recombination 11 homolog; L22, ribosomal L22 protein; TEP1, telomerase-associated protein 1. (B) The relationship of telomere length to cell division is not constant. There is a relatively constant loss of telomere length during normal cell division in the absence of compensatory mechanisms in most cells. However, telomere length can also increase with cell division in some lymphocyte subsets. Human T cells can partially sustain telomere length during cell division. Cellular stress can accelerate telomere loss rates.
Telomerase has been identified as a ribonucleoprotein enzyme that can synthesize telomeric repeats onto chromosomes. Telomerase consists of two essential components, telomerase RNA component (TERC) and telomerase reverse transcriptase (TERT) (14). In all human cells, with the exception of germ cells, stem cells, and some activated lymphocytes, telomeres lose a portion of their noncoding repetitive DNA with each cell division, and this shortening of telomeric DNA is one mechanism that can lead to cellular senescence. By contrast, there is a wide variety of evidence indicating that mice do not use replicative aging as a counting mechanism to induce senescence and to suppress the progression of cancers, as mice retain telomerase competency throughout their life span and cells from telomerase-deficient mice undergo senescence as readily as wild-type cells (101). Therefore, it seems that a telomere-independent mechanism regulates the proliferative life span of rodent cells (15), and, as discussed above, this difference between humans and mice in telomere biology presents a major shortcoming in using standard laboratory strains of mice for preclinical studies to support the development of strategies for human adoptive T cell therapy.
Telomere length in T cells. Patients with various bone marrow failure syndromes have a number of structural and functional abnormalities of telomeres and telomerase (102). Some patients with aplastic anemia have mutations in the gene encoding TERT (103), and haploinsufficiency of TERC or TERT has been associated with human dyskeratosis congenita (104) and aplastic anemia (103). Human T cells have finite clonal life spans in vitro (105), a phenomenon also manifested with other somatic tissue cells and commonly known as the Hayflick limit. In humans, lymphocytes and stem cells belong to a select class of cells that are able to induce telomerase activity (106).
For effector T cells, costimulation is required for the induction of telomerase activity (85, 107). In vitro, the limit of polyclonal expansion for human adult mature CD4+ T cells is about 30–40 doublings of the population size (84, 108). In previous studies, we found that human naive CD4+ T cells have telomeres that are, on average, 1.4 kb longer than those of human memory T cells (109). In more recent studies using CC chemokine receptor 7 (CCR7) and CD27 expression to discriminate human CD4+ memory T cell subsets, telomere length was found to differ substantially among the subsets, with CD4+CD27+CCR7+ naive T cells having the longest telomeres, followed by CD4+ CD27+CCR7– TCM cells, and then terminally differentiated CD4+CD27–CCR7– TEM cells (110). In human CD8+ memory T cell subsets, the telomeres are shorter in CD8+CD27–CD45RA+ TEM and CD8+CD27+CD45RA– TCM cell subsets than in unprimed CD8+CD27+CD45RA+ naive T cells (111). In the peripheral blood, more detailed phenotypic analysis indicates that some populations of TEM cells (CD27+CD28–CD45RA+CCR7– cells) have telomeres that are on average longer than those of CD27+CD28+CD45RA+CCR7+ naive T cells, suggesting that telomerase has forestalled telomere degradation in these cells (112). To the extent that relative preservation, or even extension, of telomere length is a biomarker of candidate stem cells, it seems that human memory stem T cells might be contained in this population.
Telomere length and adoptive T cell therapy. It is possible that previous adoptive T cell therapy clinical trials have been unsuccessful because the prolonged ex vivo culture process resulted in a population of cells that had reached, or were near, replicative senescence. Recent studies suggest that preservation of telomere length and replicative capacity correlates with the engraftment efficiency and antitumor efficacy of adoptively transferred T cell lines in patients with melanoma (86). CD28 stimulation maintained telomere length in T cells (106), and cultures that optimize costimulation might improve the engraftment and persistence of adoptively transferred T cells. Recent studies indicate that IL-15 is able to activate telomerase activity in human memory CD8+ T cells through the JAK3 and PI3K signaling pathways (113). Furthermore, IL-15 induces a sustained level of telomerase activity, and this minimizes telomere loss in memory CD8+ T cells after a substantial number of cell divisions (113, 114).
For optimal adoptive T cell therapy, it is probable that preservation of the replicative life span of memory T cells is vital for long-term immune protection. Culture methods that preserve CD28 expression on the transferred T cells might be important because CD28 expression is associated with long telomeres (32) and, following adoptive transfer, there is a correlation between CD28 expression, telomere length, and T cell persistence, with enhanced antitumor effects in patients with metastatic melanoma (86). For effector T cells that have downregulated CD28, it is possible that strategies that employ IL-7 and IL-15 during culture and/or after infusion might be beneficial (113, 114); alternatively, genetic engineering of T cells to restore CD28 expression seems to have promise to rejuvenate T cells, in which reintroduction of the CD28 gene reconstituted the ability to produce IL-2 and thereby induced autocrine proliferation after antigen stimulation (115).
One way to circumvent the problem of telomere degradation is to express TERT in human T cells. Ectopic expression of TERT in human CD8+ and CD4+ T cells leads to immortalization of these T cells (116, 117). Human T cells constitutively expressing TERT are not overtly leukemogenic, as they display minimal alterations in phenotype, specificity, and functionality and remain dependent on cytokines and antigenic stimulation for in vitro expansion. TERT reprogrammed T cells mount effective immune responses following adoptive transfer into mice bearing tumor xenografts (118). However, although in vitro studies so far indicate that constitutive TERT expression achieves immortalization without malignant transformation, caution is urged with the clinical use of life span–extended human T cells because chromosomal instability, which is associated with tumor development, has been observed in human T cells constitutively expressing TERT (119, 120).
Other strategies might forestall or reverse senescence of memory T cells. Rando and colleagues have used parabiotic pairings (i.e., a shared circulatory system) between young and old mice to demonstrate that the proliferative and regenerative capacity of aged satellite muscle cells is restored by exposure of satellite cells to young mice (121). By extension, to the extent that lymphocyte aging is extrinsically regulated, it is possible that the “parking” of memory T cells in a young environment during tissue culture could restore T cell functions. In contrast, a cell intrinsic form of senescence is mediated by the cyclin-dependent kinase inhibitor p16INK4a, as HSCs have delayed aging and improved ability to support the survival of animals in successive transplants (122). Therefore it is possible that inhibition of p16INK4a could extend the useful life span of some lymphocyte subsets.
-
Conclusion
Adoptive T cell therapy of rodent malignancies was first reported in 1955 (123), and there are no forms of FDA-approved T cell therapy for cancer available after more than 60 years of research into adoptive immunity for tumors. However, there is increasing optimism that the scientific barriers preventing clinically effective adoptive immunotherapy have been addressed. Evidence in support of the cancer stem cell hypothesis and the idea that these cells present a substantial barrier to complete tumor elimination using cytotoxic chemotherapy raises the hope that it might be possible to target these cells using adoptive T cell therapy (124). Given the success of allogeneic cellular therapy for chemotherapy-resistant hematologic malignancies (125), it is of interest to learn whether T cells can target cancer stem cells, as is suggested by the durability of responses following allogeneic T cell therapies. Advances in the understanding of T cell biology and the tumor microenvironment have provided multiple novel adoptive transfer strategies that are now poised for translation into clinical trials.
-
Acknowledgments
The author thanks Gwendolyn Binder, Anne Chew, and laboratory members for helpful discussions and is grateful for support of these studies by NIH grants 5R01CA105216, 1R01CA104679, and 5P50CA083638 and the Alliance for Cancer Gene Therapy.
Address correspondence to: Carl H. June, University of Pennsylvania, 421 Curie Blvd., Philadelphia, Pennsylvania 19104-6160, USA. Phone: (215) 573-5745; Fax: (215) 573-8590; E-mail: [email protected].
-
Footnotes
Nonstandard abbreviations used: γc, common γ chain; CCR7, CC chemokine receptor 7; MART1, melanoma-associated antigen recognized by T cells 1; TCM cell, central memory T cell; TEM cell, effector memory T cell; TERC, telomerase RNA component; TERT, telomerase reverse transcriptase.
Conflict of interest: C.H. June receives income from BD, Cell Genesys, CellDex therapuetics, and AVEO Pharmaceuticals Inc. and research support from BD, GlaxoSmithKline Inc., and Sangamo BioSciences. He also has patents and patent applications in the field of adoptive T cell therapy and immunosuppression.
Reference information: J. Clin. Invest.117:1204–1212 (2007). doi:10.1172/JCI31446.
-
References
- Southam, C.M., Brunschwig, A., Levin, A.G., Dizon, Q.S. 1966. Effect of leukocytes on transplantability of human cancer. Cancer. 19:1743-1753. View this article via: PubMed Google Scholar
- Jamieson, B.D., Ahmed, R. 1989. T cell memory. Long-term persistence of virus-specific cytotoxic T cells. J. Exp. Med. 169:1993-2005. View this article via: PubMed Google Scholar
- Michie, C.A., McLean, A., Alcock, C., Beverley, P.C. 1992. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 360:264-265. View this article via: PubMed Google Scholar
- Rosenberg, S.A., Terry, W.D. 1977. Passive immunotherapy of cancer in animals and man. Adv. Cancer Res. 25:323-388. View this article via: PubMed Google Scholar
- Greenberg, P.D. 1991. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv. Immunol. 49:281-355. View this article via: PubMed Google Scholar
- Melief, C.J. 1992. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv. Cancer Res. 58:143-175. View this article via: PubMed Google Scholar
- Mestas, J., Hughes, C.C. 2004. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172:2731-2738. View this article via: PubMed Google Scholar
- Burger, S.R. 2003. Current regulatory issues in cell and tissue therapy. Cytotherapy. 5:289-298. View this article via: PubMed Google Scholar
- van Elsas, A., Hurwitz, A.A., Allison, J.P. 1999. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190:355-366. View this article via: PubMed Google Scholar
- Attia, P., et al. 2005. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J. Clin. Oncol. 23:6043-6053. View this article via: PubMed Google Scholar
- Blansfield, J.A., et al. 2005. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J. Immunother. 28:593-598. View this article via: PubMed Google Scholar
- Boon, T., Cerottini, J.C., Van den Eynde, B., van der Bruggen, P., Van Pel, A. 1994. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 12:337-365. View this article via: PubMed Google Scholar
- Rammensee, H.G., Weinschenk, T., Gouttefangeas, C., Stevanovic, S. 2002. Towards patient-specific tumor antigen selection for vaccination. Immunol. Rev. 188:164-176. View this article via: PubMed Google Scholar
- Blackburn, E.H., Greider, C.W., Szostak, J.W. 2006. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 12:1133-1138. View this article via: PubMed Google Scholar
- Forsyth, N.R., Wright, W.E., Shay, J.W. 2002. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation. 69:188-197. View this article via: PubMed Google Scholar
- Akagi, T. 2004. Oncogenic transformation of human cells: shortcomings of rodent model systems. Trends Mol. Med. 10:542-548. View this article via: PubMed Google Scholar
- Horikawa, I., et al. 2005. Differential cis-regulation of human versus mouse TERT gene expression in vivo: identification of a human-specific repressive element. Proc. Natl. Acad. Sci. U. S. A. 102:18437-18442. View this article via: PubMed Google Scholar
- Riley, J.L., June, C.H. 2005. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 105:13-21. View this article via: PubMed Google Scholar
- Azuma, M., Phillips, J.H., Lanier, L.L. 1993. CD28- T lymphocytes. Antigenic and functional properties. J. Immunol. 150:1147-1159. View this article via: PubMed Google Scholar
- Posnett, D.N., Sinha, R., Kabak, S., Russo, C. 1994. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy.”. J. Exp. Med. 179:609-618. View this article via: PubMed Google Scholar
- Effros, R.B., et al. 1994. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence.
Exp. Gerontol. 29:601-609. View this article via: PubMed Google Scholar
- Lewis, D.E., Merched-Sauvage, M., Goronzy, J.J., Weyand, C.M., Vallejo, A.N. 2004. Tumor necrosis factor-alpha and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J. Biol. Chem. 279:29130-29138. View this article via: PubMed Google Scholar
- Weng, N.P. 2006. Aging of the immune system: How much can the adaptive immune system adapt? Immunity. 24:495-499. View this article via: PubMed Google Scholar
- Laport, G.G., et al. 2003. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 102:2004-2013. View this article via: PubMed Google Scholar
- Suntharalingam, G., et al. 2006. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355:1018-1028. View this article via: PubMed Google Scholar
- Traggiai, E., et al. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 304:104-107. View this article via: PubMed Google Scholar
- Kaech, S.M., Ahmed, R. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. View this article via: PubMed Google Scholar
- Sallusto, F., Geginat, J., Lanzavecchia, A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763.
- Fearon, D.T., Manders, P., Wagner, S.D. 2001. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 293:248-250.
- Sallusto, F., Lenig, D., Forster, R., Lipp, M., Lanzavecchia, A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708-712.
- Klebanoff, C.A., et al. 2005. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. U. S. A. 102:9571-9576.
- Monteiro, J., Batliwalla, F., Ostrer, H., Gregersen, P.K. 1996. Shortened telomeres in clonally expanded CD28–CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J. Immunol. 156:3587-3590. View this article via: PubMed Google Scholar
- Van den Hove, L.E., Van Gool, S.W., Vandenberghe, P., Boogaerts, M.A., Ceuppens, J.L. 1998. CD57+/CD28– T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia. 12:1573-1582.
- Barber, D.L., Wherry, E.J., Ahmed, R. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27-31. View this article via: PubMed Google Scholar
- Ahmed, R., Gray, D. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54-60.
- Sprent, J., Surh, C.D. 2002. T cell memory. Annu. Rev. Immunol. 20:551-579.
- Heslop, H.E., et al. 1996. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 2:551-555.
- Champagne, P., et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106-111.
- Appay, V., et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385.
- Opferman, J.T., Ober, B.T., Ashton-Rickardt, P.G. 1999. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 283:1745-1748.
- Holmes, S., He, M., Xu, T., Lee, P.P. 2005. Memory T cells have gene expression patterns intermediate between naive and effector. Proc. Natl. Acad. Sci. U. S. A. 102:5519-5523.
- Bouneaud, C., Garcia, Z., Kourilsky, P., Pannetier, C. 2005. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J. Exp. Med. 201:579-590.
- Zhang, Y., Joe, G., Hexner, E., Zhu, J., Emerson, S.G. 2005. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat. Med. 11:1299-1305.
- Kono, K., Ichihara, F., Iizuka, H., Sekikawa, T., Matsumoto, Y. 1998. Expression of signal transducing T-cell receptor zeta molecules after adoptive immunotherapy in patients with gastric and colon cancer. Int. J. Cancer. 78:301-305.
- Dudley, M.E., et al. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 298:850-854.
- Chang, A.E., et al. 1997. Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J. Clin. Oncol. 15:796-807. View this article via: PubMed Google Scholar
- Takayama, T., et al. 2000. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 356:802-807.
- Mazo, I.B., et al. 2005. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 22:259-270.
- Feuerer, M., et al. 2001. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat. Med. 7:452-458.
- Beckhove, P., et al. 2004. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J. Clin. Invest. 114:67-76.
- Schmitz-Winnenthal, F.H., et al. 2005. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 65:10079-10087.
- Choi, C., et al. 2005. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood. 105:2132-2134.
- Dhodapkar, M.V., Krasovsky, J., Olson, K. 2002. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 99:13009-13013.
- Noonan, K., et al. 2005. Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer Res. 65:2026-2034.
- Bevan, M.J. 2004. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 4:595-602.
- Ossendorp, F., Mengede, E., Camps, M., Filius, R., , and Melief, C.J.M., 1998. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J. Exp. Med. 187:693-702.
- Li, Y., Bleakley, M., Yee, C. 2005. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J. Immunol. 175:2261-2269. View this article via: PubMed Google Scholar
- Schoenberger, S.P., Toes, R.E., van der Voort, E.I., Offringa, R., Melief, C.J. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 393:480-483.
- Hung, K., et al. 1998. The central role of CD4(+) T cells in the antitumor immune response. J. Exp. Med. 188:2357-2368.
- Yee, C., et al. 2002. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U. S. A. 99:16168-16173.
- Einsele, H., et al. 2002. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 99:3916-3922.
- Rapoport, A., et al. 2005. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat. Med. 11:1230-1237.
- Miranda-Carus, M.E., Benito-Miguel, M., Llamas, M.A., Balsa, A., Martin-Mola, E. 2005. Human T cells constitutively express IL-15 that promotes ex vivo T cell homeostatic proliferation through autocrine/juxtacrine loops. J. Immunol. 175:3656-3662. View this article via: PubMed Google Scholar
- Prlic, M., Lefrancois, L., Jameson, S.C. 2002. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J. Exp. Med. 195:F49-F52.
- Nakajima, H., Shores, E.W., Noguchi, M., Leonard, W.J. 1997. The common cytokine receptor gamma chain plays an essential role in regulating lymphoid homeostasis. J. Exp. Med. 185:189-196.
- Jiang, H., Chess, L. 2004. An integrated view of suppressor T cell subsets in immunoregulation. J. Clin. Invest. 114:1198-1208.
- Shimizu, J., Yamazaki, S., Sakaguchi, S. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211-5218. View this article via: PubMed Google Scholar
- Woo, E.Y., et al. 2001. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non small cell lung cancer and late-stage ovarian cancer. Cancer Res. 61:4766-4772. View this article via: PubMed Google Scholar
- Harrington, L.E., et al. 2005. Interleukin 17-producing CD4(+) effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132.
- Langowski, J.L., et al. 2006. IL-23 promotes tumour incidence and growth. Nature. 442:461-465.
- McKenzie, B.S., Kastelein, R.A., Cua, D.J. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17-23.
- Thomas, E.D. 1999. Bone marrow transplantation: a review. Semin. Hematol. 36:95-103. View this article via: PubMed Google Scholar
- Barrett, J., and Jiang, Y.Z. 2000. Allogeneic immunotherapy for malignant diseases. Marcel Dekker Inc. New York, New York, USA. 386 pp.
- Fowler, D.H., et al. 2006. Phase I clinical trial of costimulated, IL-4 polarized donor CD4(+) T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 12:1150-1160.
- Porter, D.L., et al. 2006. A phase I trial of donor lymphocyte infusions expanded and activated ex-vivo via CD3/CD28 co-stimulation. Blood. 107:1325-1331.
- June, C.H., Blazar, B.R. 2006. Clinical application of expanded CD4+25+ cells. Semin. Immunol. 18:77-88.
- Zhang, L., et al. 2003. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348:203-213.
- Germeau, C., et al. 2005. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J. Exp. Med. 201:241-248.
- Coulie, P.G., Connerotte, T. 2005. Human tumor-specific T lymphocytes: does function matter more than number? Curr. Opin. Immunol. 17:320-325.
- Dummer, W., et al. 2002. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 110:185-192.
- Riddell, S.R., and Greenberg, P.D. 1998. Rapid expansion method (“REM”) for in vitro propagation of T lymphocytes. US Patent 5,827,642, filed October 3, 1994, and issued October 27, 1998.
- Schultze, J.L., et al. 1997. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J. Clin. Invest. 100:2757-2765.
- Kim, J.V., Latouche, J.B., Riviere, I., Sadelain, M. 2004. The ABCs of artificial antigen presentation. Nat. Biotechnol. 22:403-410.
- Levine, B.L., et al. 1997. Effects of CD28 costimulation on long term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J. Immunol. 159:5921-5930. View this article via: PubMed Google Scholar
- Weng, N.-P., Levine, B.L., June, C.H., Hodes, R.J. 1997. Regulation of telomerase RNA template expression in human T lymphocyte development and activation. J. Immunol. 158:3215-3220. View this article via: PubMed Google Scholar
- Zhou, J., et al. 2005. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J. Immunol. 175:7046-7052. View this article via: PubMed Google Scholar
- Levine, B.L., et al. 1998. Large scale production of CD4+ T cells from HIV-1-infected donors following CD3/CD28 stimulation.
J. Hematother. 7:437-448. View this article via: PubMed Google Scholar
- Kalamasz, D., et al. 2004. Optimization of human T-cell expansion ex vivo using magnetic beads conjugated with anti-CD3 and anti-CD28 antibodies. J. Immunother. 27:405-418.
- Oelke, M., et al. 2003. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig coated artificial antigen presenting cells. Nat. Med. 9:619-625.
- Luxembourg, A.T., et al. 1998. Biomagnetic isolation of antigen-specific CD8+ T cells usable in immunotherapy. Nat. Biotechnol. 16:281-285.
- Lone, Y.C., et al. 1998. In vitro induction of specific cytotoxic T lymphocytes using recombinant single-chain MHC class I/peptide complexes. J. Immunother. 21:283-294. View this article via: PubMed Google Scholar
- Dunbar, P.R., et al. 1999. Cutting edge: rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J. Immunol. 162:6959-6962. View this article via: PubMed Google Scholar
- Yee, C., Savage, P.A., Lee, P.P., Davis, M.M., Greenberg, P.D. 1999. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 162:2227-2234. View this article via: PubMed Google Scholar
- Latouche, J.B., Sadelain, M. 2000. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat. Biotechnol. 18:405-409.
- Maus, M.V., et al. 2004. Extensive replicative capacity of human central memory T cells. J. Immunol. 172:6675-6683. View this article via: PubMed Google Scholar
- Suhoski, M.M., et al. 2007. Engineering artificial antigen presenting cells to express a diverse array of co-stimulatory molecules. Mol. Ther. doi:10.1038/mt.sj.6300134.
- Hirano, N., et al. 2006. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 107:1528-1536.
- Alexander-Miller, M.A., Leggatt, G.R., Berzofsky, J.A. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 93:4102-4107.
- Yee, C., et al. 1996. Isolation of tyrosinase-specific CD8+ and CD4+ T cell clones from the peripheral blood of melanoma patients following in vitro stimulation with recombinant vaccinia virus. J. Immunol. 157:4079-4086. View this article via: PubMed Google Scholar
- Moran, T.P., et al. 2005. A novel viral system for generating antigen-specific T cells. J. Immunol. 175:3431-3438. View this article via: PubMed Google Scholar
- Blasco, M.A., et al. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 91:25-34.
- Xin, Z.T., et al. 2007. Functional characterization of natural telomerase mutations found in patients with hematological disorders. Blood. 109:524-532.
- Yamaguchi, H., et al. 2005. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 352:1413-1424.
- Vulliamy, T., et al. 2001. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 413:432-435.
- Effros, R.B., Dagarag, M., Spaulding, C., Man, J. 2005. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 205:147-157.
- Weng, N.-P., et al. 1997. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol. Rev. 160:43-54.
- Hathcock, K.S., Weng, N.P., Merica, R., Jenkins, M.K., Hodes, R. 1998. Antigen-dependent regulation of telomerase activity in murine T cells. J. Immunol. 160:5702-5706. View this article via: PubMed Google Scholar
- Pawelec, G., Rehbein, A., Haehnel, K., Merl, A., Adibzadeh, M. 1997. Human T-cell clones in long-term culture as a model of immunosenescence. Immunol. Rev. 160:31-42.
- Weng, N.-P., Levine, B.L., June, C.H., Hodes, R.J. 1995. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc. Natl. Acad. Sci. U. S. A. 92:11091-11094.
- Fritsch, R.D., et al. 2005. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J. Immunol. 175:6489-6497. View this article via: PubMed Google Scholar
- Hamann, D., et al. 1999. Evidence that human CD8(+)CD45RA(+)CD27(–) cells are induced by antigen and evolve through extensive rounds of division. Int. Immunol. 11:1027-1033.
- Rufer, N., et al. 2003. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood. 102:1779-1787.
- Li, Y., Zhi, W., Wareski, P., Weng, N.P. 2005. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J. Immunol. 174:4019-4024. View this article via: PubMed Google Scholar
- Wallace, D.L., et al. 2006. Prolonged exposure of naive CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length. Immunology. 119:243-253.
- Topp, M.S., et al. 2003. Restoration of CD28 expression in CD28–CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J. Exp. Med. 198:947-955.
- Hooijberg, E., et al. 2000. Immortalization of human CD8(+) T cell clones by ectopic expression of telomerase reverse transcriptase. J. Immunol. 165:4239-4245. View this article via: PubMed Google Scholar
- Luiten, R.M., Pene, J., Yssel, H., Spits, H. 2003. Ectopic hTERT expression extends the life span of human CD4+ helper and regulatory T-cell clones and confers resistance to oxidative stress-induced apoptosis. Blood. 101:4512-4519.
- Verra, N.C., et al. 2004. Human telomerase reverse transcriptase-transduced human cytotoxic T cells suppress the growth of human melanoma in immunodeficient mice. Cancer Res. 64:2153-2161.
- Schreurs, M.W.J., et al. 2005. Genomic stability and functional activity may be lost in telomerase-transduced human CD8+ T lymphocytes. Blood. 106:2663-2670.
- Roth, A., et al. 2005. Telomere loss, senescence, and genetic instability in CD4+ T lymphocytes overexpressing hTERT. Blood. 106:43-50.
- Conboy, I.M., et al. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 433:760-764.
- Janzen, V., et al. 2006. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 443:421-426. View this article via: PubMed Google Scholar
- Mitchison, N.A. 1955. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J. Exp. Med. 102:157-177.
- Reya, T., Morrison, S.J., Clarke, M.F., Weissman, I.L. 2001. Stem cells, cancer, and cancer stem cells. Nature. 414:105-111.
- Weiden, P.L., et al. 1979. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 300:1068-1073.
- Southam, C.M., Brunschwig, A., Levin, A.G., Dizon, Q.S. 1966. Effect of leukocytes on transplantability of human cancer. Cancer. 19:1743-1753.
-
Version history
- Version 1 (May 1, 2007): No description
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Review Series
Tumor Immunology
-
Indoleamine 2,3-dioxygenase and tumor-induced tolerance
David H. Munn et al. -
Tregs and rethinking cancer immunotherapy
Tyler J. Curiel -
DC-based cancer vaccines
Eli Gilboa -
Immune surveillance of tumors
Jeremy B. Swann et al. -
Development of TLR9 agonists for cancer therapy
Arthur M. Krieg -
Altered macrophage differentiation and immune dysfunction in tumor development
Antonio Sica et al. -
Harnessing the immune system to treat cancer
Nina Bhardwaj -
Principles of adoptive T cell cancer therapy
Carl H. June -
A cytokine-mediated link between innate immunity, inflammation, and cancer
Wan-Wan Lin et al.
Metrics



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)



