Advertisement
Research ArticlePulmonology Free access | 10.1172/JCI28295
Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function
Marco Idzko,1,2 Hamida Hammad,1 Menno van Nimwegen,1 Mirjam Kool,1 Tobias Müller,2 Thomas Soullié,1 Monique A.M. Willart,1 Daniëlle Hijdra,1 Henk C. Hoogsteden,1 and Bart N. Lambrecht1
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Idzko, M. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Hammad, H. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Nimwegen, M. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Kool, M. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Müller, T. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Soullié, T. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Willart, M. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Hijdra, D. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Hoogsteden, H. in: JCI | PubMed | Google Scholar
1Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands. 2Department of Pneumology, University of Freiburg, Freiburg, Germany.
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
Find articles by Lambrecht, B. in: JCI | PubMed | Google Scholar
Published November 1, 2006 - More info
J Clin Invest. 2006;116(11):2935–2944. https://doi.org/10.1172/JCI28295.
© 2006 The American Society for Clinical Investigation
Received: February 20, 2006; Accepted: August 29, 2006
-
Abstract
Airway DCs play a crucial role in the pathogenesis of allergic asthma, and interfering with their function could constitute a novel form of therapy. The sphingosine 1–phosphate receptor agonist FTY720 is an oral immunosuppressant that retains lymphocytes in lymph nodes and spleen, thus preventing lymphocyte migration to inflammatory sites. The accompanying lymphopenia could be a serious side effect that would preclude the use of FTY720 as an antiasthmatic drug. Here we show in a murine asthma model that local application of FTY720 via inhalation prior to or during ongoing allergen challenge suppresses Th2-dependent eosinophilic airway inflammation and bronchial hyperresponsiveness without causing lymphopenia and T cell retention in the lymph nodes. Effectiveness of local treatment was achieved by inhibition of the migration of lung DCs to the mediastinal lymph nodes, which in turn inhibited the formation of allergen-specific Th2 cells in lymph nodes. Also, FTY720-treated DCs were intrinsically less potent in activating naive and effector Th2 cells due to a reduced capacity to form stable interactions with T cells and thus to form an immunological synapse. These data support the concept that targeting the function of airway DCs with locally acting drugs is a powerful new strategy in the treatment of asthma.
-
Introduction
DCs are powerful antigen-presenting cells with a unique capacity to stimulate naive T cells (1). In the airways, immature lung DCs are ideally placed to sample inhaled antigens (2). After they acquire antigens in the lung, DCs migrate to the draining mediastinal LNs, where they localize in the T cell–rich area, and initiate immune responses. It was recently shown that induction of T cell division and effector generation is a specific function of myeloid DCs (mDCs) whereas induction of tolerance is a specialized function of lung plasmacytoid DCs (pDCs) (3–7). Not surprisingly, DCs have also been implicated in the process of Th2 sensitization that is typical of allergic asthma (2). The mDCs have additional functions in established allergic inflammation, as their conditional removal abolishes all the cardinal features of asthma, such as Th2 cytokine–dependent airway eosinophilia, goblet cell hyperplasia, and bronchial hyperreactivity (8, 9). Therefore, drugs that interfere with DC function in the airways hold great promise for being novel antiasthmatic compounds. Preferably, these drugs should be administered via inhalation to avoid systemic immunodeficiency.
The lysophospholipid growth factor sphingosine 1–phosphate (S1P) is predominantly generated by stimulated platelets and leukocytes and is present in micromolar concentration in serum and extracellular fluid (10, 11). In immune cells, S1P can modulate many different functions, including migration and cytokine and chemokine release (11, 12). The novel immunomodulator FTY720 is a chemical derivative of myriocin, a metabolite of the ascomycete Isaria sinclairii, and its phosphorylated metabolite FTY720-P is a structural homolog of S1P. FTY720 acts through 4 of the 5 G protein–coupled S1P receptors (S1PR1, S1PR3–5) (10, 11, 13). These receptors regulate cytoskeleton-dependent cellular processes, including maintenance of cellular morphology and leukocyte migration (10, 11, 13–15). FTY720 modulates lymphocyte trafficking in a dual manner: by accelerating migration into secondary lymphoid organs and blocking the egress into the medullary sinus of LNs and efferent lymphatics, leading to an overall decrease in circulating T and B lymphocytes (lymphopenia) and inhibition of lymphocyte influx into sites of inflammation (14, 16, 17). This mechanism of action makes it highly effective in suppressing transplant rejection (18–21) and T cell–mediated inflammatory diseases (14, 16).
Recently, oral treatment with FTY720 was shown to inhibit airway inflammation induced by adoptive transfer of Th1 and Th2 cells and asthma induced by active immunization and challenge with OVA (22). However, as in all other disease models of allorejection and autoimmunity, the therapeutic effects were claimed to result from T cell sequestration in lymphoid tissues, leading to peripheral lymphopenia (13). It has, however, been shown that FTY720 can also affect other cell types involved in allergic asthma, including DCs. We and others have reported that human immature and mature monocyte–derived DCs express S1PR and that S1P and FTY720 can modulate the migration, cytokine production profile, T cell stimulatory function, and maturation of human DCs in vitro (23, 24). The aim of the present study was therefore to investigate whether local treatment with FTY720 could suppress experimental asthma, focusing particularly on how it affected lymphocyte distribution and DC function.
-
Results
Local application of FTY720 and S1P during allergen challenge suppresses the cardinal features of asthma. We investigated whether local application of FTY720 or its physiological analogue S1P occurring 30 minutes prior to each allergen challenge would influence the development of eosinophilia in already sensitized mice. In these experiments, sensitization to OVA was induced using i.p. injection of OVA in the Th2 adjuvant alum (8). Control mice were sham sensitized using PBS/alum followed by challenge with OVA aerosol. As expected, OVA-sensitized mice treated with the vehicle prior to each OVA aerosol challenge developed bronchoalveolar lavage (BAL) fluid eosinophilia and lymphocytosis accompanied by enhanced Th2 cytokine production in mediastinal LNs (Figure 1, A–F). Treatment with FTY720 (Figure 1, A, C, and G) or S1P (Figure 1, B, D, and H) prior to each allergen challenge resulted in a significant reduction of the lymphocyte and eosinophil infiltrate into the BAL compartment and in peribronchial and perivascular inflammation on lung sections. The dramatic reduction of airway inflammation in FTY720- and S1P-treated mice was accompanied by significantly reduced levels of IL-4, IL-5, and IL-13 in mediastinal LNs while the concentration levels of IFN-γ and IL-10 were not significantly changed (Figure 1, C and D, and data not shown). When OVA-sensitized mice were first subjected to 3 OVA aerosol exposures and subsequently exposed to another 3 OVA or PBS exposures in the presence or absence of local FTY720 treatment, FTY720 was still able to reduce airway inflammation and Th2 cytokine production. In addition, the resolution of inflammation after stopping OVA exposure was enhanced (Figure 1J and data not shown), suggesting that FTY720 is effective in a setting mimicking therapeutic administration. In all these experiments, the local administration of FTY720 did not lead to measurable systemic levels of the drug, as measured by liquid chromatography–mass spectrometry (LC-MS) (21) (V. Brinkmann, Novartis Research Institute, Basel, Switzerland, unpublished observations).
 Figure 1
Figure 1Effect of local FTY720 and S1P treatment on asthma features. Mice were sensitized by an i.p. injection of OVA/alum on days 0 and 7. On days 19–21, mice were subjected to OVA aerosol challenges. Thirty minutes before each aerosol challenge, mice received an i.t. injection of vehicle, 0.25 μg FTY720, or S1P 10–6 M. (A and B) BAL fluid was analyzed by flow cytometry. (C and D) Mediastinal LN cell suspensions were restimulated in vitro for 4 days with OVA and assayed for cytokine production using ELISA assay. (E–H) H&E staining of lung sections of mice treated or not with FTY720 and S1P. Data in E and F are from the same experiments as in A and B, respectively. (I) FTY720 inhibits the induction of BHR. Airway obstruction to increasing doses of inhaled methacholine was measured as increase in Penh values (% increase from saline) 24 hours after the last Ag exposure. Labels indicate immunization/treatment/challenge; n = 6–8 mice per group. Data are shown as mean ± SEM. (J) Sensitized BALB/c mice were first subjected to OVA aerosol challenges on days 17–19. Subsequently, mice were subjected to an additional series of 3 daily OVA or PBS aerosol challenges on days 20–22. Thirty minutes before each of these aerosol challenges, mice received an i.t. injection of control vehicle or 0.25 μg FTY720. Twenty-four hours after the last OVA or PBS exposure, BAL was performed. Labels indicate sensitization/first series of aerosol challenges/treatment/second series of aerosol challenges. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, representative of 3 independent experiments.
One of the cardinal features of asthma is the presence of bronchial hyperresponsiveness to nonspecific stimuli, such as inhaled methacholine. As shown in Figure 1I, OVA challenge of OVA-sensitized mice induced a significant change in responsiveness to methacholine compared with that of sham-sensitized mice. Over the complete dose range of inhaled methacholine, a higher average enhanced pause (Penh) value was measured using whole-body plethysmography in actively immunized and challenged mice, but this bronchial hyperresponsiveness was significantly attenuated by local treatment with FTY720. In S1P-treated mice, there was no attenuation of bronchial hyperresponsiveness (data not shown), most likely due to the direct bronchoconstricting effects of S1P acting on the S1PR2 on bronchial smooth muscle cells (25).
Effect of local FTY720 treatment on lymphocyte distribution. A systemic treatment with FTY720 in mice acts by selectively sequestering lymphocytes into secondary lymphoid organs (14, 17, 21). Therefore we next examined whether the intratracheal (i.t.) administration of FTY720 affected systemic lymphocyte distribution in vivo. Circulating CD3+CD4+ lymphocytes, CD3+CD8+ lymphocytes, and CD19+ B cells were analyzed 24 hours after the last FTY720 administration to OVA-sensitized and -challenged mice. As shown in Figure 2A, the local administration of FTY720 did not cause any T or B blood lymphopenia. The distribution of these cell types in mediastinal LNs and nondraining axillary and inguinal LNs was also addressed at the same time point. Strikingly, local FTY720 administration did not affect the number of CD4+ T lymphocytes, CD8+ T lymphocytes, or CD19+ B lymphocytes in the nondraining peripheral nodes (Figure 2B) whereas it markedly reduced the number of these cells in mediastinal LNs (Figure 2C).
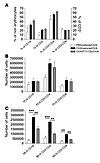 Figure 2
Figure 2Effect of local FTY720 treatment on number of lymphocytes in peripheral blood (A), peripheral LNs (B) and lung-draining LNs (C). Single-cell suspensions from blood, peripheral LNs, and lung-draining LNs were made and stained for the presence of CD4+ or CD8+ T cells and CD19+ B cells. Labels indicate sensitization/treatment/challenge. Treatment consisted of 0.25 μg of FTY720 or vehicle. Data were calculated as percentage of nonerythrocytes (A) or as absolute cell numbers (B and C). MLN, mediastinal LN; PLN, peripheral LN. Data indicate mean ± SEM. **P < 0.01; ***P < 0.001, representative of 3 independent experiments.
Effect of local FTY720 treatment on blood, LN, and lung DCs. As the local treatment with FTY720 was effective in reducing asthma features without sequestering lymphocytes in secondary lymphoid organs, we hypothesized that direct alteration of DC function might be responsible for the treatment effect. To support our hypothesis, we addressed the expression of S1PRs on sorted lung CD11c+ major histocompatibility complex II+ (CD11c+MHCII+) DCs, meticulously excluding CD11c+ autofluorescent alveolar macrophages. As shown in Figure 3, lung DCs expressed all S1PR1–5 subtypes using RT-PCR amplification. Overall, the expression of S1P4 was the most pronounced.
 Figure 3
Figure 3Expression of S1PRs on lung DCs. RT-PCR analysis of S1PR (S1PR1–5) expression was performed on highly purified, freshly isolated lung DCs. Far left and far right columns show 100-bp DNA ladder.
Systemic treatment with FTY720 has been shown to enhance the number of circulating DCs (26). As shown in Figure 4A, OVA challenge in OVA-sensitized mice led to an increase in circulating total DCs and mDCs compared with those in sham-sensitized mice. However, the number of circulating total MHCIIhighCD11chigh DCs and MHCIIhighCD11chighCD11bhigh mDCs 24 hours after the last local FTY720 treatment was unaltered in FTY720-treated compared with vehicle-treated mice. Allergen challenge of actively sensitized mice similarly led to a strong increase in the number of DCs in draining mediastinal LNs compared with those in sham-sensitized mice (27, 28). After FTY720 treatment, the number of CD11c+MHCII+ DCs was not altered in peripheral LNs (Figure 4B) whereas in mediastinal LNs (Figure 4C), the number was strongly reduced.
 Figure 4
Figure 4Effect of local FTY720 treatment on the number of DCs in peripheral blood (A), peripheral LNs (B), and lung-draining LNs (C). Single-cell suspensions from blood, peripheral, and mediastinal LNs of PBS/vehicle/OVA- or OVA/vehicle/OVA- or OVA/FTY720/OVA-treated mice were prepared. The cells were stained with anti-CD11c and anti-MHCII and analyzed by flow cytometry. Autofluorescent and PI-positive dead cells were excluded from the gate. Data were calculated as absolute number of cells. Data indicate mean ± SEM. **P < 0.01; ***P < 0.001, representative experiments out of 3.
We next investigated the effect of FTY720 treatment on lung DC maturation. In FTY720-treated animals, there were no significant differences in the expression of the DC maturation markers CD40, CD80, CD83, or CD86 on lung or LN DCs compared with that in vehicle-treated mice. When lung-derived DCs were stained intracellularly for IL-12 and TNF-α, there was a 1-log reduction in the level of IL-12 whereas TNF-α was unaffected by FTY720 treatment (data not shown).
Effect of local FTY720 treatment on migration of lung DCs to the mediastinal nodes. The reduction in the total number of DCs in the draining LNs in FTY720-treated mice suggested an effect on the migration capacity of lung DCs in vivo. However, a reduction in DC numbers in mediastinal LNs could also result from a reduction in lung inflammation and, concomitantly, a reduction in DC influx into LNs. As previously reported, the transport of i.t.-injected FITC-OVA is a function of lung-derived DCs, and this assay can be used to study the effect of drugs on steady state DC migration (7). The number of MHCII+CD11c+ DCs carrying fluorescent FITC-OVA cargo was enumerated in the mediastinal LNs 24 hours after instillation, and as shown in Figure 5A, concomitant treatment with 0.25 μg FTY720 significantly inhibited the migration of lung DCs to the LNs. At the same time, significantly more FITC+ DCs were retained in the lungs after FTY720 treatment (Figure 5B). These data also show that FTY720 is not toxic to lung DCs but rather retains them in the lung. On an individual cell basis, there was no difference in the intensity of FITC-OVA staining in lung or LN DCs exposed to FTY720 or not, illustrating that the drug did not interfere with antigen (Ag) uptake. In vitro experiments revealed further that FTY720-treated DCs were still able to migrate in response to the CCR7 agonist chemokine CCL19 but no longer migrated in response to S1P (Supplemental Figure 1; available online with this article; doi:10.1172/JCI28295DS1), suggesting that a loss of chemotactic responsiveness to LN-expressed S1P was the primary cause of reduced migration of DCs to the draining nodes.
 Figure 5
Figure 5Effect of local FTY720 administration on lung DC migration to the lung-draining LNs. (A) On day 0, mice were instilled i.t. with 10 mg/ml FITC-OVA with or without 0.25 μg FTY720. Twenty-four hours later, the presence of FITC+ migrating DCs in thoracic draining LNs was analyzed by flow cytometry. (B) Effect of FTY720 on lung DCs. Twenty-four hours after installation of FITC-OVA+/vehicle, lungs were enzymatically digested and stained for the presence of FITC+MHCII+CD11c+ DCs. Experiments were repeated 3 times with similar results; n = 4 mice per group. Data indicate mean ± SEM. *P < 0.05; **P < 0.01.
Effect of FTY720 on Th2 priming induced by lung mDCs. Increased migration of DCs to the draining LNs is an integral part of both the primary and secondary immune response to inhaled allergens, and it has been shown that this migration is essential for generating Th2 effector function from resting T cells in LNs (9, 27–29). To find out whether local FTY720 treatment can also suppress development of new Th2 effector cells in response to inhaled OVA, we made use of a model in which tolerogenic pDCs are first removed from the system through treatment with a pDC-depleting Ab, GR1-Ab (3). As previously reported, when 800 μg of OVA was given i.t. to naive mice with normal numbers of pDCs (isotype control treatment), this did not lead to Th2 priming, and a subsequent allergen challenge 10 days later did not lead to airway eosinophilia (Figure 6). However, when pDCs were depleted with anti-GR1, i.t. administration of OVA led to Th2 priming, as exemplified by strong airway eosinophilia and lymphocytosis in the BAL fluid and eosinophilia and goblet cell hyperplasia in the lung upon allergen challenge to the lung (Figure 6, A and B).
 Figure 6
Figure 6Local administration of FTY720 prevents asthma development driven by endogenous mDCs. On day 0, mice received an i.t. injection of OVA in the presence or absence of FTY720. From days –1 to 2, mice were injected i.p. with anti-GR1 Abs to deplete tolerogenic pDCs or isotype control Abs. Ten days later, mice were subjected to 3 OVA aerosol challenges. (A) BAL fluid was analyzed by flow cytometry. (B) H&E staining of lung sections. (C) MLN cells restimulated in vitro for 4 days with OVA and assayed by ELISA. Data indicate mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, representative of 2 independent experiments.
When the first i.t. OVA-priming dose was admixed with FTY720 in pDC-depleted mice, the number of eosinophils and lymphocytes were strongly reduced in BAL fluid (Figure 6A) and lung tissues (Figure 6B) compared with those in mice receiving vehicle admixed OVA. In addition, the generation of OVA-specific effector Th2 cells in the mediastinal nodes in pDC-depleted mice was also suppressed by FTY720 (Figure 6C)
Effect of in vitro FTY720 treatment on potential of mDCs to induce Th2 development in vivo. In the experiments described above, the inhibitory effects of FTY720 on Th2 sensitization could also be due to influences on structural airway cells. We therefore performed experiments in which mDCs were treated ex vivo with FTY720 prior to i.t. transfer in vivo. As the number of DCs obtained from the lungs of mice was too small to perform large adoptive transfer experiments, we decided to use BM-derived DCs (BMDCs). Similarly to the in vivo effects on lung DCs, the maturation state (MHCII, CD40, CD80, and CD86) of OVA-pulsed BMDCs was not affected by prior overnight incubation with FTY720 (data not shown). As previously reported, adoptive i.t. transfer of OVA-pulsed mDCs leads to Th2 priming, and eosinophilic airway inflammation subsequently develops upon OVA aerosol challenge 10 days later (6). As expected, in mice receiving unpulsed DCs, only a few inflammatory cells were observed in the BAL fluid (Figure 7, A and B). Mice immunized with OVA-pulsed DCs demonstrated a strong cellular recruitment of lymphocytes and eosinophils in the BAL fluid (Figure 7A) and peribronchial and perivascular area (Figure 7B) while goblet cell hyperplasia was induced. The pretreatment of OVA-pulsed DCs (OVA-DCs) with FTY720 ex vivo significantly inhibited in a dose dependent manner (data not shown) the development of these salient features of asthma. This was accompanied by a significant decrease in the levels of IL-4, IL-5, and IL-13 in the mediastinal LNs even though the concentration of IL-10 was not significantly changed (Figure 7C and data not shown). Although production of IFN-γ was reduced, this change failed to reach statistical significance. The same inhibitory effects on DC-driven Th2 development and tissue inflammation were found for pretreatment of BMDCs with 10–6 M S1P (data not shown).
 Figure 7
Figure 7Effects of in vitro FTY720 treatment on the potential of mDCs to induce asthmatic inflammation. On day 0, mice received an i.t. injection of vehicle/OVA-DCs, FTY720/OVA-DCs, or unpulsed DCs. From days 10–13, mice were subjected to OVA aerosol challenges. (A) BAL fluid was analyzed by flow cytometry. (B) H&E staining of lung sections showing induction of peribronchial and perivascular inflammation and goblet cell hyperplasia in vehicle/OVA-DC–immunized mice. (C) Mediastinal LN cells were restimulated in vitro for 4 days with OVA, and cytokines were measured using ELISA. Data indicate mean ± SEM. **P < 0.01; ***P < 0.001, representative of 3 experiments.
Effect of in vitro FTY720 treatment on the capacity of DCs to activate and polarize Ag-specific T cells in vitro. The effects of local FTY720 treatment in inhibiting mDC-driven Th2 sensitization may be due in part to inhibition of DC migration to mediastinal LNs but may also result from an inherent effect of FTY720 on the stimulatory or polarizing capacity of DCs. To more directly study the effect of S1PR ligation on T cell differentiation in the absence of any confounding effects of altered DC migration, we tested the effect of FTY720 and S1P on BMDC-driven T cell proliferation of TCR Tg (DO11.10), OVA-specific T cells in vitro. FTY720- and S1P-treated OVA-DCs induced a lower proliferation of OVA-specific naive T cells compared with vehicle-treated OVA-DCs (Figure 8A). Moreover, the T cells stimulated by FTY720- or S1P-treated OVA-DCs produced lower levels of Th2 cytokines than T cells stimulated with vehicle-treated OVA-DCs (Figure 8, B and C). T cells cocultured with OVA-DCs that were treated with the highest concentration of FTY720 and S1P also showed a decrease in the levels of IFN-γ compared with T cells stimulated with untreated OVA-DCs.
 Figure 8
Figure 8Effect of in vitro FTY720 and S1P treatment on the capacity of DCs to activate and polarize Ag-specific T cells in vitro. BMDCs were pulsed or not with 100 μg/ml of OVA overnight. DCs were also treated for 30 minutes with FTY720 (FTY720/OVA-DCs) or S1P before addition of OVA (S1P/OVA-DCs). DCs were collected and cocultured for 4 days with naive OVA-specific CD4+ T cells (A–C) or in vitro–differentiated Th2 cells (D). (A) Cell proliferation was assessed by [3H] thymidine (1 μCi/well) uptake in a 16-hour pulse. Data indicate mean ± SEM for triplicate cultures. (B–D) Cytokines were measured in supernatants by ELISA. Data indicate mean ± SEM. *P < 0.05.
In separate experiments, FTY720-treated DCs were also cocultured with in vitro–differentiated DO11.10 Th2 cells, obtained by stimulating LN cells with OVA in the presence of IL-4, anti–IFN-γ, and anti–IL-12 (30). As shown in Figure 8D, pretreatment of BMDCs with FTY720 also strongly inhibited the production of Th2 cytokines by stimulated effector Th2 cells. Similar results were obtained when CD11c+ DCs were sorted from the lungs of OVA-exposed mice and put into culture with sorted lung polyclonal CD4+ T cells from OVA-exposed mice. Again, FTY720 treatment inhibited the production of Th2 cytokines.
Effect of in vitro FTY720 treatment on the capacity of DCs to form stable interactions with T cells. FTY720 has been shown to interfere with the levels and activation of the Rho family of small GTPases that regulate the cytoskeletal proteins. These GTPases are involved in formation of dendrites and stable interactions between T cells and DCs (31). OVA-specific T cells obtained from TCR Tg OTII mice carrying the Vα2 TCR chain were cultured for 40 minutes with MHCII-positive DCs and formed cellular interactions that became considerably longer when DCs were pulsed with OVA protein and were abolished when DCs were pretreated with FTY720 prior to coculture (Figure 9A). Gradually, over time, in the case of T cells interacting with DCs, the intensity of surface TCR expression was higher in areas contacting each MHCII+ DC compared with areas of the T cell not directly contacting an MHCII+ DC, which is highly suggestive of the formation of an immunological synapse. This synapse formation was abolished by pretreatment of DCs with FTY720 or by coculture with unpulsed DCs (Figure 9B). This was not due to the reduced capacity of FTY720-treated DCs to process whole OVA protein, as OVA-peptide–pulsed DCs also formed less stable interactions when treated with FTY720 (Figure 9A).
 Figure 9
Figure 9Effect of in vitro FTY720 treatment of mDCs on capacity to form stable interactions with antigen-specific T cells. DCs were pulsed or not with OVA protein or peptide (OVA323–339) in the presence or absence of FTY720. Live DCs were labeled with anti-MHCII (green) and imaged by confocal microscopy. (A) Contact duration between MHCII-labeled unpulsed or OVA-pulsed DCs treated or not with FTY720 and TCR-labeled OVA-specific naive T cells was measured in vitro using time-lapse confocal microscopy. Data are shown as mean ± SEM. *P < 0.05. (B) Time-lapse microscopy images showing TCR-labeled OVA-specific TCR Tg T cells (anti-vα2l; red) interacting with MHCII-labeled DCs (M5/114-FITC; green) show concentration of TCR at the site of contact with OVA-bearing DCs, leading to a synapse (yellow).
-
Discussion
Th2 lymphocytes play a crucial role in allergic asthma, and strategies aimed at interfering with the function of these cells or individual cytokines (anti–IL-5, soluble IL-4 receptor) have been proposed as novel forms of therapy (32). Recently, the immunomodulator FTY720 has been shown to be effective as an oral drug for the treatment of experimental asthma, induced by adoptive transfer of effector Th2 cells (22). In addition, numerous other Th1-mediated experimental disease models of graft rejection, type 1 diabetes mellitus, autoimmunity, and arthritis have been successfully treated using FTY720, and this drug is currently employed in phase III clinical trials for prevention of allograft rejection (18, 20). In all of these models, FTY720 has been administered systemically where its major mechanism of action is the inhibition of lymphocyte egress from the LN medulla, resulting in peripheral lymphopenia (17, 19, 21). S1PR agonists cause sequestration of lymphocytes in secondary lymphoid tissues by acting as functional antagonists that downregulate S1PR1s on lymphocytes or by preventing entry of medullary lymphocytes into the efferent lymphatics for recirculation (17). Although FTY720 does not impair the activation, expansion, and memory function of lymphocytes (33), the occurrence of peripheral lymphopenia and, as a consequence, the reduced recruitment of effector lymphocytes to sites of inflammation might constitute a form of immunodeficiency, with its inherent risk of developing opportunistic infections, which is undesirable for the treatment of non–life threatening diseases, such as allergic asthma. To avoid this systemic immunodeficiency, we tested to determine whether local i.t. treatment with FTY720 was similarly able to suppress the cardinal features of asthma. Local FTY720 treatment given prior to each allergen challenge in sensitized mice was able to inhibit the salient pathologies of asthma, including eosinophilic inflammation, goblet cell hyperplasia, Th2 cytokine production, and most importantly, bronchial hyperreactivity to inhaled methacholine, even when the treatment was delayed until after the 3 first aerosol challenges. FTY720 most likely acted on S1PRs, as the antiinflammatory effect was mimicked by S1P; however, the precise receptor subtype involved is unclear. Our preliminary data using a selective S1P1R agonist, SEW2871, suggest that agonism of S1P1R alone is sufficient to inhibit allergic airway inflammation (unpublished observations). In striking contrast to oral FTY720 treatment, with treatment via inhalation we observed no peripheral blood lymphopenia or sequestration of T and B lymphocytes in the secondary lymphoid organs. In addition, lung administration did not lead to measurable plasma FTY720 levels. There was, however, a reduction in allergen-induced BAL fluid lymphocytosis and a reduction in the number of T and B lymphocytes in the lung-draining mediastinal LNs, most likely due to the reduction in airway inflammation in FTY720-treated mice, and a concomitant reduced influx of lung-derived CCR7+ effector Th2 lymphocytes to LNs. Migration of effector lymphocytes to the lung-draining LNs does occur in the lungs of allergen-challenged mice and is CCR7 dependent (34).
DCs are crucial in both the initiation and maintenance phase of allergic asthma and therefore constitute ideal targets for the development of novel antiasthmatic drugs (2). As a proof of concept, selective depletion of DCs during allergen challenge abolishes all the cardinal features of asthma, including bronchial hyperreactivity (30). DCs are crucial because they can locally activate Th2 effector cells in the airway wall by providing chemotactic cues for Th2 cells (CCL17 and CCL22) and by delivering MHC and costimulatory signals (4, 35–37). Additionally, at times of allergen challenge, DCs migrate from the site of allergic inflammation to mediastinal LNs to stimulate new rounds of division in recirculating central memory cells or from naive T cells, thus feeding the inflammatory response with new waves of effector cells (2, 27–29). As FTY720 and S1P suppressed inflammation without altering lymphocyte distribution, we studied the effect of FTY720 and S1P on lung DCs and found that freshly isolated lung DCs express all S1PR subtypes, consistent with prior reports on cultured DCs (23, 26). One of the striking observations we made after local FTY720 treatment was a reduction in the number of mDCs in mediastinal LNs of allergen-challenged mice. Although, again, a reduction in lung inflammation might be responsible for reduced input of immigrating lung-derived DCs in LNs, we also observed a reduction in DC migration to LNs in naive mice when FTY720 was administered together with fluorescently labeled OVA, concomitant with an accumulation of DCs in the lung compartment. The same effect of systemically administered FTY720 and S1P agonists was seen on DC migration from the skin following FITC skin painting (26, 38).
We and others have shown that S1P agonists and FTY720 alter the regulation of actin-dependent cytoskeletal processes in DCs by downstream signaling of different S1PRs through small Rho family GTPases, such as Rho and Rac/Cdc42 (23, 38). It was shown that a correct balance and possibly cyclic activity of Rho and Rac/Cdc42 are essential for DC migration to occur and that this process might be inhibited by FTY720 (38). However, this explanation seemed less likely as we performed in vitro chemotactic assays. DC migration from the periphery of the lung to the mediastinal nodes is CCR7 dependent (39). It has been shown by Lan et al. that FTY720 treatment of DCs impairs CCR7-dependent chemotactic migration to CCL19 across endothelial cells in vitro (26) whereas Czeloth et al. showed that migration to the other CCR7 ligand, CCL21, across Transwell inserts was unaffected (38). In our experiments, we found that FTY720 treatment of DCs did not affect migration toward CCR7 ligands in vitro whereas, clearly, responsiveness to the chemotactic signal S1P was reduced. We therefore favor the idea that FTY720 specifically suppresses migration of DCs by downregulation of the S1PR1 and not by interference with the actin cytoskeleton.
Inhalation of low-endotoxin OVA is a normally tolerogenic event in which pDCs inhibit the potential of mDCs to prime for effector Th2 cells (3–5). By depleting pDCs, this tolerogenic response is turned into robust Th2 priming by mDCs. In this model, FTY720 completely abolished the development of Th2 effector cells, and consequently, asthma did not develop upon repeated OVA challenge. There may be several reasons for the lack of Th2 priming. First, S1P agonists have been shown to alter the function and stability of bronchial epithelial tight junctions (40). These tightened junctions could be a barrier to the uptake of inhaled OVA, thus leading to a lack of Th2 priming. To exclude this possibility, we also incubated mDCs with FTY720 or S1P prior to adoptive transfer to naive mice. This pretreatment of DCs significantly reduced their potential to induce Th2 priming, and consequently, asthmatic inflammation did not develop. Therefore, the effects of FTY720 are most consistent with an autonomous defect on DCs.
Second, FTY720 inhibited the migration of lung-derived DCs to LNs, thus inhibiting proper T cell priming. Priming of recirculating naive T cells or reactivation of central memory T cells in LNs depends on input of DCs from the periphery of the lung in a process that is CCR7 dependent (39). However, inhibition by FTY720 of DC-mediated FITC-OVA transport was not absolute, and the DCs that escape FTY720 inhibition might still induce T cell activation. This indeed was the case when we followed primary T cell activation in a cohort of adoptively transferred DO11.10 T cells in vivo (data not shown). Even in FTY720-treated mice, OVA inhalation still led to T cell divisions in mediastinal nodes, although the strength of the T cell response was reduced. We therefore favor the third possibility that FTY720- or S1P-treated DCs are also defective in priming naive and memory T cells due to an intrinsic defect in T cell stimulatory capacity. Consistent with this, we cultured OVA-pulsed BMDCs with OVA-specific T cells from naive mice and observed a strong and dose-dependent reduction in proliferation and production of both Th1 and Th2 effector cytokines when DCs were preincubated with FTY720. In addition, FTY720 treatment of DCs inhibited their potential to stimulate primed effector Th2 cells generated under polarized Th2 conditions in vitro. Similar effects of FTY720 and S1P were noticed on the immunostimulatory capacity of human monocyte–derived DCs matured using LPS although in the human setting, Th1 cytokines were preferentially inhibited while Th2 priming was essentially weak but unaffected (23, 24). Our results showed that local FTY720 treatment inhibits IL-12 production in lung DCs, a finding similar to that in human data.
FTY720 might modify the potential of DCs to stimulate T cells in several ways. The induction and maintenance of effector cytokine production in T cells in the lungs depends upon an adequate antigen-processing function and maturation of lung DCs (41). Surprisingly, neither FTY720 nor S1P altered the expression of CD40, CD80, CD83, CD86, programmed death ligand-1 (PDL-1), PDL-2, and MHCII in immature or mature BMDCs (data not shown). Moreover, local FTY720 treatment did not alter the maturation of lung and LN DCs. These data are in accordance with studies in which S1P agonism did not affect maturation of murine and human DCs (23, 24, 26). FTY720 treatment of DCs could also interfere with their potential to form stable and long-lasting interactions with T cells in the LNs or lung parenchyma. The small Rho family GTPases (such as Rac1 and Rac2) are modified by FTY720, and expression of these factors in DCs has been shown to be essential for the occurrence of DC–T cell interaction (31). In accordance, FTY720 treatment of DCs indeed abolished the formation of long-lasting DC–T cell interactions typical of an immunological synapse that is critical for induction of cellular immunity (31). Whether local treatment with FTY720 would also reduce the formation of an immunological synapse between recirculating T cells and lung DCs in the airways or lung parenchyma remains to be shown.
In conclusion, the present study demonstrates for what we believe is the first time that the local administration of FTY720 inhibits Th2-mediated cardinal features of asthma by altering the function of lung DCs without causing any systemic lymphopenia. These findings could pave the way for a feasibility study in which this compound or a more selective S1PR agonist is administered via aerosol to patients with asthma. This paper validates the concept that targeting airway DC function is a powerful method to treat asthma.
-
Methods
Mice. BALB/c and C57BL/6 mice (6–8 weeks old) were purchased from Harlan. OVA-TCR Tg mice (DO11.10 BALB/c and OTII C57BL/6) were bred at the Erasmus Medical Center. All experiments were subject to review by an independent Animal Ethics Committee that approved all experimentation (Stichting DEC Consult, Utrecht, The Netherlands).
A mouse model of asthma induced by systemic i.p. sensitization. BALB/c mice (n = 6–8 per group) were sensitized to OVA by i.p. injection of OVA/alum (10 μg OVA grade V adsorbed to 1 mg aluminium hydroxide; Sigma-Aldrich) on days 0 and 7 and were subjected to OVA aerosol challenges (grade III) on days 17–19; aerosol challenges were dispensed from a jet nebulizer delivering 1% OVA in PBS for 30 minutes. Thirty minutes before each OVA exposure, mice were anesthetized using Avertin (Sigma-Aldrich) and received an i.t. injection of control vehicle, of 0.25 μg FTY720, or of 10–6 M S1P in a volume of 80 μl. Twenty-four hours after the last OVA exposure, BAL was performed and lungs and LNs were resected and digested using collagenase/DNAse, as described (7, 27).
A mouse model of asthma induced by endogenous myeloid airway DCs. In order to study the effect of FTY720 on the function of endogenous airway mDCs, mice were first depleted of tolerogenic pDCs by 3 i.p. injections of the pDC-depleting antibody GR1 (RB6-8C5) on days –1, 0, and 1 as described (3). On day 0, 800 μg of OVA (LPS low; Worthington Biochemical Corp.) was injected i.t., admixed with 0.25 μg of FTY720 or vehicle. After a washout period of 10 days, mice were subjected to 3 OVA aerosol challenges of 30 minutes on each of 3 consecutive days.
A mouse model of asthma induced by adoptive transfer of BMDCs. We have developed a model in which sensitization to inhaled OVA is induced by i.t. injection of OVA-pulsed BM-derived mDCs (6). DCs were prepared as previously described, by growing BM cells in medium containing recombinant mouse GM-CSF. At day 9 of culture, cells were pulsed overnight with 100 μg/ml LPS–low OVA (Worthington Biochemical Corp.). Cells were treated with 1 μM of S1P dissolved in 4% BSA, the indicated concentration of FTY720 (0.025–2.5 μg), or vehicle 30 minutes before addition of OVA. After antigen pulsing, nonadherent DCs were collected, washed to remove free OVA or S1PR agonists, and resuspended in PBS at a concentration of 12.5 × 106 cells/ml.
For in vivo experiments, BALB/c mice were anesthetized on day 0 with Avertin (2% v/v in PBS), and 1 × 106 vehicle DCs, OVA-DCs, FTY720-OVA-DCs or S1P-OVA-DCs were instilled through the opening vocal cords, as described (6). On days 10–12, mice were exposed to a 30-minute OVA aerosol challenge. Mice were sacrificed 24 hours after the last aerosol challenge.
Flow cytometry and sorting. BAL cells were stained for 30 minutes with anti–I-Ad/I-Ed FITC (macrophages/DCs), anti-CCR3 PE (eosinophils), anti-CD3 and anti-CD19 cychrome (lymphocytes) (eBioscience), and anti-CD11c APCs (macrophages/DCs) in PBS containing 0.5% BSA and 0.01% sodium azide. Differential cell counts were analyzed by flow cytometry, as previously described (42).
For determination of systemic lymphocyte distribution, heparinized blood samples (200 μl) were lysed using hypotonic ammonium chloride buffer and stained for T cell subsets (FITC–anti-CD3, PE–anti-CD4, Cychrome–anti CD8), B cells (PE–anti-CD19) or DCs (FITC–anti-MHCII, APC–anti-CD11c, PE–anti-CD11b). Peripheral (axillary and inguinal) and mediastinal LN cells were stained with the same combination. Absolute cell number was calculated by multiplying the total leukocyte number by the percentage (%) of each subset.
For analysis of DC maturation, BM, lung, or LN cell suspensions were stained with FITC-labeled anti–I-Ad/I-Ed, with PE-labeled anti-CD40, anti-CD80, anti-CD83, and anti-CD86, and with APC-labeled anti-CD11c antibodies. For sorting of DCs from lung suspensions, nonautofluorescent CD11c+, MHCII+CCR3–CD3–CD19– cells were sorted using a FacsARIA flow cytometer (BD) as described (42).
To address migration of lung DCs, 80 μl of FITC-OVA (10 mg/ml) with or without 0.25 μg FTY720 or control DMSO vehicle was administered i.t. through the opening vocal cords using an 18-gauge polyurethane catheter connected to the outlet of a micropipette as previously described (7). At 24–36 hours after injection, migrating DCs were enumerated in mediastinal LNs as CD11c+MHCII+ cells carrying FITC+ material.
In all experiments, dead cells were excluded from analysis using propidium iodide. Analysis was performed on a FACSCalibur flow cytometer (BD), using CellQuest (version 3.3; BD) and FlowJo software (version 6.4.7; Tree Star Inc.).
Expression of S1PRs on lung DCs. Lung DCs were sorted using flow cytometry, and total RNA was extracted from the cells using Tri zol Reagent (Invitrogen). To obtain cDNA, 5 μg of total RNA was primed with oligo-8 dT primers (Hermann Synthetische Biomoleküle) and reverse transcribed with StrataScript Reverse Transcriptase (Stratagene). Primers for the different murine S1PRs were designed to fit published sequence data (43). PCR was performed using 10 μl of iQ Supermix (Bio-Rad), 7 μl H2O, 1 μl of each primer (final concentration 0.5 μM each), and 1 μl cDNA. Amplification conditions were as follows: 12 minutes initial denaturation at 95°C followed by 45 cycles of denaturation (94°C) for 30 seconds, annealing (57°C S1PR1–4, 62°C S1PR5) for 30 seconds, and amplification (72°C) for 45 seconds. PCR products were separated by electrophoresis on a 2.5% agarose gel and visualized by ethidium bromide staining.
Determination of BHR. Twenty-four hours after the last OVA aerosol challenge, nonspecific airway responsiveness was measured by exposing awake mice to aerosolized PBS to set a baseline value, followed by increasing concentrations of aerosolized methacholine (1.5625, 3.125, 6.25, 12.5, and 25 mg/ml in PBS for 3 minutes; Sigma-Aldrich) using ultrasonic nebulizers. Penh values were measured for 3 minutes after each methacholine aerosol using a whole-body plethysmograph (Buxco). The average Penh values were expressed for each methacholine concentration as the percentage increase over baseline Penh values (44).
Activation of OVA-specific naive and effector T cells by mDCs. Naive CD4+ T cells (1 × 105) were purified from unmanipulated DO11.10 TCR Tg (H-2d) mice and were cocultured with BMDCs (1 × 104), which were pretreated with the indicated concentrations of FTY720, S1P, or vehicle alone and 10 μg/ml OVA protein in round-bottom 96-well tissue culture plates. In separate experiments, naive DO11.10 T cells were first differentiated for 7 days into effector Th2 cells in the presence of IL-4, anti–IFN-γ, and anti IL-12 as previously described (30). After washing, these effector Th2 cells were stimulated with DCs as for naive T cells. After 4 days, supernatants were harvested and analyzed for the presence of IFN-γ, IL-4, IL-5, IL-13, and IL-10 cytokines by ELISA. In separate wells, proliferation was measured by pulsing with [3H]thymidine (1 μCi/well) for the last 16 hours of culture, followed by measurement of uptake in a beta counter (TopCount; Packard; GMI). Results are given as mean cpm ± SEM of triplicate cultures.
To visualize dynamic interactions between DCs and specific T cells, OVA-specific T cells obtained from OTII TCR Tg (H-2b) mice were cocultured with unpulsed BMDCs, OVA protein–pulsed BMDCs (100 μg/ml), or OVA peptide–pulsed BMDCs (1 μg/ml) treated or not with 0.25 μg FTY720 overnight in an imaging chamber at 37°C in an atmosphere of 5% CO2. Prior to incubation, DCs were stained with an FITC-labeled antibody to I-Ak,b (M5/114), and T cells were stained with biotin-tagged TCR Vα2 antibodies, followed by Qdot 655 streptavidin conjugates (Invitrogen). Interactions were visualized with an LSM 510 confocal microscope (Zeiss) equipped with 488-nm and 633-nm lasers. Time-lapse images were collected every 20 seconds for 40 minutes. Interactions were quantified using Imaris software (version 5.0; Bitplane AG).
Cytokine measurements. To measure cytokine levels, mediastinal LN cells were plated in round-bottom 96-well plates (1 × 106 cells/ml) and restimulated with OVA (10 μg/ml) for 4 days. The presence of IL-4, IL-5, IL-10, IL-13, and IFN-γ was assayed on supernatants by ELISA (BD).
Statistics. For all experiments, the statistical significance of differences between samples was calculated using the Mann-Whitney U test for unpaired data. Differences were considered significant at P < 0.05.
-
Acknowledgments
This work was supported by a Netherlands Organization of Scientific Research Vidi grant (to B.N. Lambrecht) and a Veni grant (to H. Hammad) and by an Emmy Noether Fellowship from the Deutsche Forschungsgemeinschaft (DFG ID7/3-1 to M. Idzko).
Address correspondence to: Bart N. Lambrecht, Department of Pulmonary Medicine, Room Ee2251a, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. Phone: 31-10-408-7703; Fax: 31-10-408-9453; E-mail: [email protected].
-
Footnotes
Nonstandard abbreviations used: Ag, antigen; BAL, bronchoalveolar lavage; BMDC, BM-derived DC; i.t., intratracheal(ly); mDC, myeloid DC; MHCII, major histocompatibility complex II; OVA-DCs, OVA-pulsed DCs; pDC, plasmacytoid DC; Penh, enhanced pause; S1P, sphingosine 1–phosphate; S1PR, S1P receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J. Clin. Invest.116:2935–2944 (2006). doi:10.1172/JCI28295
Marco Idzko and Hamida Hammad contributed equally to this work.
-
References
- Banchereau, J., Steinman, R.M. 1998. Dendritic cells and the control of immunity. Nature. 392:245-252.
- Lambrecht, B.N., Hammad, H. 2003. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 3:994-1003.
- De Heer, H.J., et al. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89-98.
- Kohl, J., et al. 2006. A regulatory role for the C5a anaphylatoxin on type 2 immunity in asthma. J. Clin. Invest. 116:783-796.
- Lambrecht, B.N. 2006. An unexpected role for the anaphylatoxin C5a receptor in allergic sensitization. J. Clin. Invest. 116:628-632.
- Lambrecht, B.N., et al. 2000. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 106:551-559.
- Vermaelen, K.Y., Carro-Muino, I., Lambrecht, B.N., Pauwels, R.A. 2001. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193:51-60.
- Lambrecht, B.N., Salomon, B., Klatzmann, D., Pauwels, R.A. 1998. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J. Immunol. 160:4090-4097. View this article via: PubMed Google Scholar
- Van Rijt, L.S., Lambrecht, B.N. 2005. Dendritic cells in asthma: a function beyond sensitization. Clin. Exp. Allergy. 35:1125-1134.
- Ishii, I., Fukushima, N., Ye, X., Chun, J. 2004. Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. 73:321-354.
- Spiegel, S., Milstien, S. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4:397-407.
- Graeler, M., Goetzl, E.J. 2002. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. Faseb J. 16:1874-1878.
- Rosen, H., Sanna, G., Alfonso, C. 2003. Egress: a receptor-regulated step in lymphocyte trafficking. Immunol. Rev. 195:160-177.
- Matloubian, M., et al. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355-360.
- Rosen, H., Goetzl, E.J. 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5:560-570.
- Graler, M.H., Goetzl, E.J. 2004. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. Faseb J. 18:551-553. View this article via: PubMed Google Scholar
- Wei, S.H., et al. 2005. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat. Immunol. 6:1228-1235.
- Bohler, T., et al. 2000. FTY 720A mediates reduction of lymphocyte counts in human renal allograft recipients by an apoptosis-independent mechanism. Transpl. Int. 13(Suppl. 1):S311-S313.
- Brinkmann, V., Pinschewer, D.D., Feng, L., Chen, S. 2001. FTY720: altered lymphocyte traffic results in allograft protection. Transplantation. 72:764-769.
- Budde, K., et al. 2002. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J. Am. Soc. Nephrol. 13:1073-1083. View this article via: PubMed Google Scholar
- Mandala, S., et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296:346-349.
- Sawicka, E., et al. 2003. Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J. Immunol. 171:6206-6214. View this article via: PubMed Google Scholar
- Idzko, M., et al. 2002. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. Faseb J. 16:625-627. View this article via: PubMed Google Scholar
- Muller, H., et al. 2005. The immunomodulator FTY720 interferes with effector functions of human monocyte-derived dendritic cells. Eur. J. Immunol. 35:533-545.
- Rosenfeldt, H.M., et al. 2003. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. Faseb J. 17:1789-1799.
- Lan, Y.Y., et al. 2005. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am. J. Transplant. 5:2649-2659.
- Van Rijt, L.S., et al. 2002. Allergen-induced accumulation of airway dendritic cells is supported by an increase in CD31(hi)Ly-6C(neg) bone marrow precursors in a mouse model of asthma. Blood. 100:3663-3671.
- Vermaelen, K., Pauwels, R. 2003. Accelerated airway dendritic cell maturation, trafficking, and elimination in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 29:405-409.
- Harris, N.L., Watt, V., Ronchese, F., Le Gros, G. 2002. Differential T cell function and fate in lymph node and nonlymphoid tissues. J. Exp. Med. 195:317-326.
- Van Rijt, L.S., et al. 2005. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201:981-991.
- Benvenuti, F., et al. 2004. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 305:1150-1153.
- Barnes, P.J. 2004. New drugs for asthma. Nat. Rev. Drug Discov. 3:831-844.
- Pinschewer, D.D., et al. 2000. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J. Immunol. 164:5761-5770. View this article via: PubMed Google Scholar
- Bromley, S.K., Thomas, S.Y., Luster, A.D. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6:895-901.
- Huh, J.C., et al. 2003. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J. Exp. Med. 198:19-30.
- Vermaelen, K.Y., et al. 2003. Matrix metalloproteinase-9-mediated dendritic cell recruitment into the airways is a critical step in a mouse model of asthma. J. Immunol. 171:1016-1022. View this article via: PubMed Google Scholar
- Zhou, B., et al. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6:1047-1053.
- Czeloth, N., Bernhardt, G., Hofmann, F., Genth, H., Forster, R. 2005. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 175:2960-2967. View this article via: PubMed Google Scholar
- Hammad, H., et al. 2002. Monocyte-derived dendritic cells induce a house dust mite-specific Th2 allergic inflammation in the lung of humanized SCID mice: involvement of CCR7. J. Immunol. 169:1524-1534. View this article via: PubMed Google Scholar
- Gon, Y., et al. 2005. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc. Natl. Acad. Sci. U. S. A. 102:9270-9275.
- Van Rijt, L.S., et al. 2004. Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of TH2 effector responses in a mouse model of asthma. J. Allergy Clin. Immunol. 114:166-173.
- Van Rijt, L.S., et al. 2004. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J. Immunol. Methods. 288:111-121.
- Jolly, P.S., et al. 2004. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 199:959-970.
- Hamelmann, E., et al. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766-775.. View this article via: PubMed Google Scholar
-
Version history
- Version 1 (November 1, 2006): No description



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)









