ISSN: 1837-9664Journal of Cancer
J Cancer 2014; 5(7):590-597. doi:10.7150/jca.9413 This issue Cite
Research Paper
Transcatheter Arterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma: Clinical Outcome and Safety in Elderly Patients
Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, Osaka 543-0027, Japan.
Abstract
Aim: The aim of our study was to compare clinical outcomes between elderly patients aged ≥75 years (elderly group, n=66) with intermediate hepatocellular carcinoma (HCC) undergoing transcatheter arterial chemoembolization (TACE) and younger patients aged <75 years (control group, n=84) with intermediate HCC undergoing TACE.
Methods: Clinical outcomes, including overall survival (OS) and tumor response rate at initial therapy, were compared between these two groups.
Results: The median survival time and the 1- and 3-year cumulative OS rates were 2.90 years and 84.1% and 48.0%, respectively, in the elderly group and 2.44 years and 78.2% and 39.3%, respectively, in the control group (p=0.887). The objective response rate in the elderly group was 81.8% (54/66 patients), while that in the control group was 78.6% (66/84 patients) (p=0.227).
Conclusion: Elderly patients with intermediate HCC undergoing TACE had a prognosis comparable with that of younger patients with intermediate HCC undergoing TACE.
Keywords: transcatheter arterial chemoembolization, intermediate hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a major health problem. It is the fifth most common type of cancer worldwide and the third most common cause of cancer-related death (1-3). The prognosis for untreated HCC is poor in general, and the curative treatments for this disease comprise surgical resection, radiofrequency ablation, and liver transplantation (1-3). Noncurative therapies for HCC include transcatheter arterial chemoembolization (TACE), radioembolization, molecular targeting therapies such as sorafenib, and radiation therapy (1-8).
Societal aging implies that the number of elderly patients with malignancy will rise in the future (9). In Japan, 75-year-old men and women have an average expected life span of around 5 and 10 years, respectively, and Japan has the greatest longevity in the world (10). The risk of developing HCC is known to be age-dependent, and patients aged ≥75 years sometimes present with HCC (11, 12). The increased longevity of the population means that more elderly patients with HCC are to be expected in the coming years. In Japan, the adjusted HCC mortality has increased in recent years (13). Moreover, the average age of patients with HCC in Japan is increasing, as is the proportion of elderly patients with HCC (14). Thus, there is an urgent need to identify the optimal management for HCC in elderly patients.
TACE is a procedure whereby an embolic agent is injected into the tumor-feeding artery to deprive the tumor of its major nutrient source by means of embolization; this results in ischemic necrosis of the targeted tumor (8). The survival benefit of TACE in patients with unresectable HCC was established in two randomized controlled trials and one meta-analysis (15-17). Thus, TACE plays an important role in the treatment of unresectable HCC. It is clearly defined as a first-line therapy with a better 2-year survival rate than that of conservative therapy (18). The Barcelona Clinic Liver Cancer (BCLC) intermediate stage (BCLC-B) includes Child-Pugh A and B patients with multifocal HCC, defined as more than three tumors of any size or two to three tumors with a maximal diameter of ≤3 cm and a single HCC of ≤5 cm (18-21). The BCLC classification indicates that these patients are optimal candidates for TACE (18, 19).
Advanced age was previously considered to be a contraindication for TACE in the treatment of HCC (22). There are few data regarding the clinical outcome in elderly patients with intermediate HCC undergoing TACE (21, 23-26) and most of them are reported from countries other than Japan. Furthermore, the BCLC classification does not stratify strategies according to age (18, 19). Whether elderly patients with intermediate HCC who undergo TACE have a prognosis comparable with that of younger patients with intermediate HCC who undergo TACE therefore remains elusive (21, 23-25). The aim of the present study was to compare clinical outcomes between elderly patients with intermediate HCC undergoing TACE and younger patients with intermediate HCC undergoing TACE.
Materials and Methods
Patients. We performed TACE as an initial treatment in 150 treatment-naive patients diagnosed with intermediate-stage HCC in the Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, Japan between December 2003 and December 2012. Of these patients, 147 were treated with TACE using an epirubicin-mitomycin-lipiodol (EML) emulsion, and three were treated with TACE using a miriplatin-lipiodol emulsion. We categorized them into two groups: the elderly group (≥75 years old, n=66) and the control group (<75 years old, n=84). The breakpoint of 75 years of age was chosen because in Japan, patients aged ≥75 years are covered by a health insurance system that differs from that for patients aged <75 years. We compared the clinical outcomes including overall survival (OS), tumor response rate, and safety between these two groups. Patients diagnosed with HCC rupture at initial therapy were not included in this study because they were treated with transcatheter arterial embolization without chemoembolization.
Written informed consent was obtained from all patients prior to each therapy, and the study protocol complied with all provisions of the Declaration of Helsinki. This study was approved by the Ethics Committee of Osaka Red Cross Hospital, Japan, and the need for written informed consent was waived because the data were analyzed retrospectively and anonymously. The present study comprised a retrospective analysis of patient records registered in our database, and all treatments were conducted in an open-label manner.
HCC diagnosis. HCC was diagnosed using abdominal ultrasound and dynamic computed tomography (CT) scans (hyperattenuation during the arterial phase in all or some part of the tumor and hypoattenuation in the portal-venous phase) and/or magnetic resonance imaging (MRI), based mainly on the recommendations of the American Association for the Study of Liver Diseases (18). Arterial- and portal-phase dynamic CT images were obtained at approximately 30 and 120 s, respectively, after the injection of the contrast material. When carrying out angiography, we also confirmed the presence of intermediate-stage HCC using CT during hepatic arteriography (CTHA) and CT during arterial portography (CTAP) (27, 28).
TACE procedure. In our angiography room, a catheter was advanced to the superior mesenteric artery, and CTAP was performed to investigate the site and size of the HCC. Furthermore, we confirmed the patency of the portal vein at the time of postmesenteric portography. A catheter was then advanced to the celiac artery, and a microcatheter was advanced to the common hepatic artery or proper hepatic artery through a catheter. This approach was used to perform CTHA and digital subtraction angiography with the purpose of investigating the tumor vascularity and identifying the feeding vessels. After the completion of these procedures, a microcatheter was advanced as close as possible to the feeding vessels of the targeted tumor. This was followed by intra-arterial infusion of an anticancer agent and lipiodol emulsion via the feeding arteries according to tumor size and liver function (20, 29, 30). After the infusion of the anticancer agent and lipiodol emulsion, gelatin sponge particles were slowly injected into the feeding arteries to prevent reflux into untreated segments. The sites of injection of the embolizing agents were segmental or subsegmental in all patients treated with TACE. When patients had poor liver function, the doses of the anticancer agents and lipiodol were reduced.
Assessment of treatment efficacy. Treatment efficacy was evaluated using CT findings within 2 months after the initial treatment. We regarded lipiodol accumulation in targeted tumors seen on CT scans as an indication of necrosis. This was because several studies previously reported that the lipiodol retention areas observed on CT corresponded to necrotic areas (31, 32). A complete response (CR) was defined as the disappearance of all targeted tumors or 100% tumor necrosis, a partial response (PR) was defined as a ≥50% reduction in tumor size and/or necrosis, and progressive disease (PD) was defined as ˃25% tumor enlargement and/or the appearance of any new HCC tumors. Stable disease (SD) was defined as disease that did not qualify for classification as CR, PR, or PD.
Follow-up. Follow-up after each therapy comprised periodic blood tests and monitoring of tumor markers, including α-fetoprotein and des-γ-carboxy prothrombin. Dynamic CT scans and/or MRI were obtained every 2 to 4 months after each therapy. Chest CT, whole abdominal CT, brain MRI, and bone scintigraphy were performed when extrahepatic HCC recurrence was suspected. When disease progression of the treated HCC lesions was observed after the initial therapy and/or new hepatic lesions were observed, the most appropriate treatments were performed if the liver functional reserve was adequate and if patients did not refuse such therapies. These treatments included transcatheter arterial therapies in most cases. However, when the treated lesion was well controlled after the initial therapy and the new lesion appeared in the liver, percutaneous ablative therapies were also considered. In cases that were refractory to transcatheter arterial therapies or those involving extrahepatic metastases, a molecular targeting therapy such as sorafenib was also considered (33).
Statistical analysis. Data were analyzed using univariate and multivariate analyses. Continuous variables were compared using the unpaired t-test, and categorical variables were compared using Fisher's exact test. For analysis of OS, follow-up ended at the time of death from any cause, and the remaining patients were censored at the last follow-up visit. The cumulative OS rates were calculated using the Kaplan-Meier method and tested using the log-rank test. Factors with a p value of <0.05 in univariate analysis were subjected to multivariate analysis using the Cox proportional hazards model. These statistical methods were used to estimate the interval from initial treatment. Data were analyzed using SPSS software (SPSS Inc., Chicago, IL, USA) for Microsoft Windows. Data are expressed as the mean ± standard deviation. Values of p<0.05 were considered to be statistically significant.
Results
Baseline characteristics. The baseline characteristics of the patients in the two groups are shown in Table I. The median observation periods were 1.6 years (range, 0.2-5.3 years) in the elderly group and 1.9 years (range, 0.2-9.0 years) in the control group. There was a significantly higher proportion of female patients, a lower positivity rate for hepatitis B surface antigen, and a lower body mass index (BMI) in the elderly group. The serum albumin level, prothrombin time (PT), and platelet count were significantly higher in the elderly group than in the control group, and the proportion of patients with Child-Pugh class A disease was significantly higher in the elderly group than in the control group. These findings indicated that patients in the elderly group had a liver functional reserve superior to that of patients in the control group. No significant difference was observed in comorbid diseases between the two groups.
Baseline characteristics between the elderly group and the control group.
| Variables | Elderly group (n=66) | Control group (n=84) | P value |
|---|---|---|---|
| Age (years) | 80.8 ± 4.2 | 65.7 ± 5.6 | <0.001a |
| Gender, male/female | 34 / 32 | 63 / 21 | 0.003b |
| Maximum tumor size (cm) | 5.7 ± 2.8 | 5.1 ± 3.2 | 0.220a |
| Tumor distribution, bilobar/unilobar | 23 / 43 | 37 / 47 | 0.314b |
| Tumor number, >5 vs. ≤5 | 14 / 52 | 21 / 63 | 0.698b |
| Child-Pugh classification | |||
| Child-Pugh A / B | 53 / 13 | 52 / 32 | 0.019b |
| Causes of liver disease | |||
| B/C/non B and non C/B and C | 0 / 47 / 19 / 0 | 13 / 49 / 21 / 1 | 0.003b |
| Efficacy of initial TACE | |||
| CR/PR/SD/PD | 10/44/11/1 | 20/46/18/0 | 0.227b |
| AST (IU/L) | 58.2 ± 29.6 | 63.8 ± 34.0 | 0.289a |
| ALT (IU/L) | 43.5 ± 27.9 | 52.9 ± 37.7 | 0.095a |
| ALP (IU/L) | 383.6 ± 193.4 | 439.1 ± 235.8 | 0.124a |
| GGT (IU/L) | 119.0 ± 182.6 | 157.3 ± 211.1 | 0.244a |
| Serum albumin (g/dL) | 3.78 ± 0.48 | 3.53 ± 0.52 | 0.003a |
| Total bilirubin (mg/dL) | 0.95 ± 0.64 | 1.15 ± 0.96 | 0.151a |
| Prothrombin time (%) | 88.4 ± 18.4 | 81.0 ± 17.0 | 0.012a |
| Platelets (×104/mm3) | 15.6 ± 7.7 | 12.4 ± 6.5 | 0.007a |
| AFP (ng/mL) | 1367.6 ± 3614.3 | 1250.8 ± 3679.8 | 0.846a |
| DCP (mAU/mL) | 7046.4 ± 19775.1 | 10076.1 ± 42352.1 | 0.563a |
| Duration of hospitalization (days) | 10.7 ± 5.0 | 12.6 ± 6.7 | 0.053a |
| Body mass index (kg/m2) | 21.8 ± 3.7 | 24.6 ± 3.6 | <0.001a |
| Comorbid diseases | |||
| Hypertension, yes/no | 44 / 22 | 43 / 41 | 0.068b |
| Cardiovascular disease, yes/no | 14 / 52 | 12 / 72 | 0.285b |
| Respiratory disease, yes/no | 4 / 62 | 7 / 77 | 0.756b |
| Cerebrovascular disease, yes/no | 11 / 55 | 13 / 71 | >0.999b |
| Diabetes mellitus, yes/no | 19 / 47 | 35 / 49 | 0.124b |
| Serum creatinine (mg/dL) | 0.98 ± 0.49 | 0.90 ± 0.37 | 0.270a |
Data are expressed as number or mean ± standard deviation. TACE; transcatheter arterial chemoembolization, CR; complete response, PR; partial response, SD; stable disease, PD; progressive disease, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a; unpaired t test, b; Fisher,s exact test.
Median survival time (MST) and cumulative overall survival (OS). The MST and the 1-, 3-, and 5-year cumulative OS rates were 2.90 years and 84.1%, 48.0%, and 15.0%, respectively, in the elderly group and 2.44 years and 78.2%, 39.3%, and 33.8%, respectively, in the control group (p=0.887). MST: median survival time.

Median survival time and cumulative OS rates. The median survival time (MST) and the 1-, 3-, and 5-year cumulative OS rates were 2.90 years and 84.1%, 48.0%, and 15.0%, respectively, in the elderly group and 2.44 years and 78.2%, 39.3%, and 33.8%, respectively, in the control group; there was no significant difference between the two groups (p=0.887) (Figure 1).
Mean doses of anticancer agents and lipiodol in the two groups. In the elderly group, TACE using EML emulsion containing epirubicin (Farmorubicin; Pfizer) at a mean dose of 39.1 ± 9.8 mg, mitomycin (Mitomycin C; Kyowa Hakko Kirin Company, Ltd., Tokyo, Japan) at a mean dose of 8.8 ± 3.3 mg, and lipiodol at a mean dose of 5.9 ± 2.8 ml was performed in 65 patients, and TACE using miriplatin-lipiodol emulsion containing miriplatin (Miripla; Dainippon Sumitomo, Tokyo, Japan) at a dose of 140 mg and lipiodol at a dose of 7 ml was performed in 1 patient (29, 30, 34). In the control group, TACE using EML emulsion containing epirubicin at a mean dose of 39.3 ± 10.9 mg, mitomycin at a mean dose of 8.9 ± 3.1 mg, and lipiodol at a mean dose of 5.6 ± 2.7 ml was performed in 82 patients, and TACE using miriplatin-lipiodol emulsion containing miriplatin at a dose of 120 mg and lipiodol at a dose of 6 ml was performed in 2 patients (29, 30, 34).
Treatment efficacy at initial treatment in the two groups. In the elderly group, a CR was achieved in 10 patients, a PR in 44 patients, SD in 11 patients, and PD in 1 patient. Thus, the objective response rate (ORR) in the elderly group was 81.8% (54/66 patients). In the control group, a CR was achieved in 20 patients, a PR in 46 patients, SD in 18 patients, and PD in 0 patients. Thus, the ORR in the TACE group was 78.6% (66/84 patients). The difference in initial treatment efficacy between the two groups did not reach significance (p=0.227).
Univariate and multivariate analyses of factors contributing to OS. Univariate analysis identified the following factors as being significantly associated with OS for all cases (n=150): the Child-Pugh classification (p<0.001), tumor number of ≤5 (p=0.001), tumor distribution (p=0.001), maximum tumor size of ≤4.5 cm (p=0.008), objective tumor response at initial treatment (p=0.004), serum albumin level of ≥3.7 g/dl (p=0.014), and total bilirubin level of ≥1.0 mg/dl (p=0.011) (Table II). The hazard ratios and 95% confidence intervals calculated using multivariate analysis for the eight factors with p-values of <0.05 in the univariate analysis are detailed in Table II. The Child-Pugh classification (p=0.039), tumor number of ≤5 (p=0.018), maximum tumor size of ≤4.5 cm (p=0.048), and ORR at initial therapy (p=0.010) were found to be significant predictors linked to OS in multivariate analysis.
Causes of death. Thirty-two patients in the elderly group (48.5%) died during the follow-up period. The causes of death were HCC progression in 24 patients, liver failure in 4 patients, and miscellaneous causes in 4 patients. Fifty-two patients in the control group (61.9%) died during the follow-up period, and the causes of death were HCC progression in 29 patients, liver failure in 19 patients, and miscellaneous causes in 4 patients.
Adverse events and hospitalization days in the two groups. In both groups, symptoms associated with postembolization syndrome such as fever, appetite loss, abdominal pain, and nausea were transient and mostly resolved within 2 weeks after initial treatment (35). In the elderly group, serious adverse events (SAEs) were observed in three patients (4.5%). Each of these three patients had one of the following SAEs: cholangitis, aspiration pneumonia, or liver abscess formation. All of these SAEs were managed successfully. Thus, TACE-related mortality in the elderly group was 0%. In the control group, SAEs were observed in five patients (6.0%). Each of these five patients had one of the following SAEs: acute respiratory distress syndrome (ARDS), hepatic encephalopathy, hyponatremia, hyperbilirubinemia, or refractory ascites. All of these SAEs were managed successfully, although in one patient who developed ARDS, management in the intensive care unit was required. Thus, TACE-related mortality in the control group was 0%. The mean number of hospitalization days in the elderly group tended to be less than that in the control group (10.7 ± 5.0 vs. 12.6 ± 6.7 days, respectively; p=0.053).
Univariate and multivariate analysis contributing to overall survival.
| Variables | n | Univariate Analysis | Multivariate Analysis | |
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P valuea | |||
| Gender, male vs. female | 97 / 53 | 0.952 | ||
| Age (years), ≥75 vs. <75 | 66 / 84 | 0.887 | ||
| Child-Pugh, A vs. B | 105 / 45 | <0.001 | 0.524 (0.283-0.969) | 0.039 |
| Tumor number, >5 vs. ≤5 | 35 / 115 | 0.001 | 0.489 (0.270-0.885) | 0.018 |
| Tumor distribution, bilobar vs. unilobar | 60 / 90 | 0.001 | 1.128 (0.632-2.016) | 0.683 |
| Maximum tumor size, ≥4.5cm vs. <4.5cm | 73 / 77 | 0.008 | 0.633 (0.402-0.996) | 0.048 |
| Objective response at initial therapy, yes / no | 120 / 30 | 0.004 | 2.017(1.180-3.448) | 0.010 |
| AST (IU / L), ≥50 vs. < 50 | 83 / 67 | 0.188 | ||
| ALT (IU / L), ≥ 40 vs. < 40 | 77 / 73 | 0.992 | ||
| ALP (IU / L), ≥ 360 vs. < 360 | 73 / 77 | 0.060 | ||
| GGT (IU / L), ≥ 80 vs. < 80 | 73 / 77 | 0.322 | ||
| Serum albumin level (g / dL), ≥3.7 vs. <3.7 | 74 / 76 | 0.014 | 1.219 (0.690-2.154) | 0.496 |
| Total bilirubin (mg / dL), ≥1.0 vs. <1.0 | 59 / 91 | 0.011 | 0.766 (0.472-1.243) | 0.281 |
| Platelet count (×104 / mm3), ≥13 vs. <13 | 73 / 77 | 0.937 | ||
| Prothrombin time (%), ≥87 vs. <87 | 73 / 77 | 0.070 | ||
| Serum creatinine (mg / dL), ≥1.0 vs. <1.0 | 45 / 105 | 0.926 | ||
| Diabetes mellitus, yes/no | 54/96 | 0.423 | ||
| Body mass index (kg/m2), ≥23 vs. <23 | 74/76 | 0.835 | ||
| Serum AFP (ng / mL), ≥40 vs. <40 | 72 / 78 | 0.166 | ||
| DCP (mAU / mL), ≥800 vs. <800 | 79 / 71 | 0.069 | ||
CI; confidence interval, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP, alpha-fetoprotein; DCP, des-γ-carboxy prothrombin, a; Cox proportional hazard model.
Subgroup analyses according to the Child-Pugh classification. No significant difference (p=0.390) was observed between the two groups in terms of OS in patients with Child-Pugh class A disease (53 patients [80.3%] in the elderly group and 52 [61.9%] in the control group); the MST was 3.62 years in the elderly group and 3.32 years in the control group (A). Similarly, there was no significant difference (p=0.434) between the two groups in terms of OS in patients with Child-Pugh class B disease (13 patients [19.7%] in the elderly group and 32 [38.1%] in the control group); the MST was 2.10 years in the elderly group and 1.72 years in the control group (B). MST: median survival time.
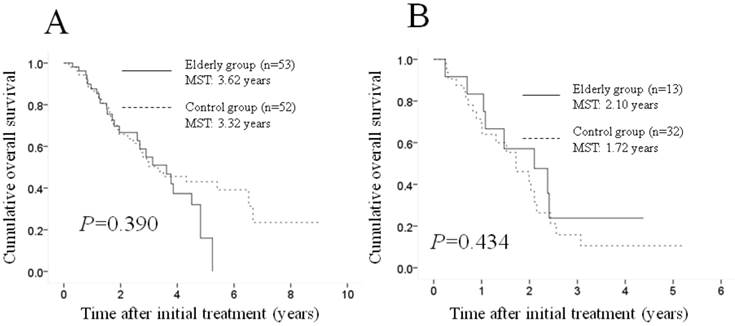
Subgroup analyses according to the Child-Pugh classification. A significant difference was observed between the two groups in terms of the Child-Pugh classification (p=0.019), and we therefore performed subgroup analyses according to this classification. No significant difference (p=0.390) was observed between the two groups in terms of OS in patients with Child-Pugh class A disease (53 patients [80.3%] in the elderly group and 52 patients [61.9%] in the control group); the MST was 3.62 years in the elderly group and 3.32 years in the control group (Figure 2A). Similarly, no significant difference (p=0.434) was found between the two groups in terms of OS in patients with Child-Pugh class B disease (13 patients [19.7%] in the elderly group and 32 [38.1%] in the control group); the MST was 2.10 years in the elderly group and 1.72 years in the control group (Figure 2B).
Subgroup analyses according to maximum tumor size. Although there were no significant differences in baseline tumor characteristics between the two groups, tumor-related characteristics are reportedly prognostic factors associated with OS in patients with HCC undergoing TACE (8, 15-17). Hence, we performed subgroup analyses according to maximum tumor size. No significant difference (p=0.861) was observed between the two groups in terms OS in patients with a maximum tumor size of ≥4.5 cm (39 patients [59.1%] in the elderly group and 38 [45.2%] in the control group); the MST was 2.41 years in the elderly group and 1.88 years in the control group (Figure 3A). Similarly, no significant difference (p=0.559) was found between the two groups in terms of OS in patients with a maximum tumor size of <4.5 cm (27 patients [40.9%] in the elderly group and 46 [54.8%] in the control group); the MST was 3.78 years in the elderly group and 2.92 years in the control group (Figure 3B).
Subgroup analyses according to gender and other factors. Since a significant difference of proportion of male patients was observed between the two groups, we performed subgroup analyses according to gender. In male patients (34 patients in the elderly group and 63 in the control group), the MST was 2.68 years in the elderly group and 2.56 years in the control group (p=0.986). In female patients (32 patients in the elderly group and 21 in the control group), the MST was 3.62 years in the elderly group and 2.10 years in the control group (p=0.885). In subgroup analyses of other factors [tumor number (>5 or ≤5), presence or absence of ORR, tumor distribution (bilobar or unilobar), pretreatment serum albumin level (≥3.7g/dl or <3.7g/dl) and total bilirubin (≥1mg/dl or <1mg/dl)], no significant difference was observed in the two groups in terms of OS (data not shown).
Discussion
In Japan, there is a trend toward an increasing number of elderly patients with HCC. In addition, the latest estimates suggest that the incidence of HCC peaks above the age of 70 years worldwide (36). However, few investigators have reported the clinical outcome in elderly patients with intermediate-stage HCC who underwent TACE as initial therapy, although there are several studies on the clinical outcome in elderly patients with HCC who underwent surgical resection or ablative therapies (21, 23-26, 37-47). Hence, we conducted the current comparative study.
Our results showed no significant difference in OS or treatment efficacy at initial therapy between the elderly group and the control group, and similar results were obtained in all subgroup analyses. These findings indicate that elderly patients with intermediate-stage HCC who underwent TACE had a prognosis comparable with that of younger patients. Cohen et al. reported that the MST in patients with HCC aged ≥75 years treated with TACE was 1.88 years, while in our study, the MST in the elderly group was 2.90 years (25). Because the baseline characteristics differed between their study and ours, it may not be possible to reach a definitive conclusion. However, our TACE procedure may have been more effective than that of Cohen et al.
Subgroup analyses according to maximum tumor size. No significant difference (p=0.861) was observed between the two groups in terms of OS in patients with a maximum tumor size of ≥4.5 cm (39 patients [59.1%] in the elderly group and 38 [45.2%] in the control group); the MST was 2.41 years in the elderly group and 1.88 years in the control group (A). Similarly, there was no significant difference (p=0.559) between the two groups in terms of OS in patients with a maximum tumor size of <4.5 cm (27 patients [40.9%] in the elderly group and 46 [54.8%] in the control group); the MST was 3.78 years in the elderly group and 2.92 years in the control group (B). MST: median survival time.

In the present study, a significantly higher proportion of female patients and a lower positivity rate for hepatitis B surface antigen were found in the elderly group than in the control group and patients in the elderly group had a liver functional reserve superior to that of patients in the control group. In previous studies, elderly patients with HCC were more likely to be women (37-47). This may have been associated with a larger female elderly population because of their longer life expectancy (39). The fact that male tend to drink and smoke more than female in general may also be associated with our observations, although in this study, drinking history and smoking history are not exactly taken from all studied subjects. Furthermore, as in our study, elderly patients with HCC were more likely to have hepatitis C virus (HCV) than hepatitis B virus (HBV) carriers in many previous studies (37-47). This finding may be explained by the fact that most HBV carriers acquire the virus via vertical transmission in the perinatal period, whereas most HCV carriers are infected at a later stage in life. HCC therefore manifests as a complication in HCV carriers much later in life than in HBV carriers (40-47). Interestingly, however, the elderly group had a significantly lower BMI than that of the control group. Hepatic steatosis is significantly correlated with an increasing BMI and results in accelerated liver carcinogenesis (48-50). These facts may be related to our observations.
In our multivariate analysis, the Child-Pugh classification, tumor number of ≤5, maximum tumor size of ≤4.5cm, and ORR at initial therapy were significant predictors associated with OS. Takayasu et al. reported in their large study that the degree of liver damage, alpha-fetoprotein level, maximum tumor size, number of lesions, and degree of portal vein invasion were significant factors linked to OS according to their multivariate analysis (8). Our results are consistent with their reports.
In general, elderly patients have a significantly higher proportion of comorbid diseases than younger patients (24, 25, 37-47) However, our baseline characteristics showed no significant difference in comorbid diseases between the two groups. Elderly patients with HCC with severe or numerous comorbid diseases may be excluded from the current analysis because of the expected TACE-related SAEs. The fact that the proportion of patients with Child-Pugh B disease was significantly lower in the elderly group than in the control group may be also due to the same reason.
In our study, TACE-related mortality was 0% in both groups. TACE-related mortality reportedly ranges from 0.5% to 7% (8, 21, 23). Furthermore, the mean number of hospitalization days in the elderly group tended to be fewer than that in the control group. Our safety profile of TACE in elderly patients with HCC is encouraging.
This study had several limitations. First, it was a retrospective study over the period of 10 years. Thus, diagnostic procedure and treatment procedure for HCC may not be consistent in each patient, leading to bias. Second, the sample sizes in the two cohorts were small for analysis. Third, as mentioned earlier, elderly patients with severe comorbid diseases may be excluded from this analysis, also potentially leading to bias. Larger prospective comparative studies will therefore be needed in the future to confirm these results. However, our study results demonstrated that the elderly group had a prognosis comparable with that in the control group and that our TACE procedure was safe.
In conclusion, elderly patients with intermediate-stage HCC undergoing TACE had a prognosis comparable with that of younger patients with intermediate-stage HCC undergoing TACE. TACE for elderly patients with intermediate-stage HCC should not be withheld based on advanced age alone.
Acknowledgements
The authors would like to thank Haruko Takada for data collection.
Competing Interests
The authors have not received any financial support for this study and have no conflicts of interest to declare.
References
1. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273
2. de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol 56 Suppl. 2012;1:S75-S87
3. Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22-29
4. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390
5. Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010;25(4):664-671
6. Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, Spain J, Srinivas S, Shrikanthan S, Aucejo FN, Kim R, Menon KV. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(12):1697-1705
7. Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, Denys A, Lee C. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197(4):W562-570
8. Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469
9. Hankey BF, Ries LA, Kosary CL, Feuer EJ, Merrill RM, Clegg LX, Edwards BK. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. 2000;11:31-35
10. Abridged life tables for Japan 2006. Ministry of Health, Labour and Welfare. http://www.mhlw.go.jp/english/database/db-hw/lifetb06/index.html
11. Cho SJ, Yoon JH, Hwang SS, Lee HS. Do young hepatocellular carcinoma patients with relatively good liver function have poorer outcomes than elderly patients? J Gastroenterol Hepatol. 2007;22:1226-1231
12. Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, Nakanishi H, Itakura J, Takahashi Y, Kurosaki M, Enomoto N, Izumi N. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518-527
13. Kiyosawa K, Tanaka E. Characteristics of hepatocellular carcinoma in Japan. Oncology 62 Suppl. 2002;1:5-7
14. Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, Monden M, Kudo M; The Liver Cancer Study Group of Japan. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676-691
15. Llovet JM, Real MI, Montaña X. et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734-1739
16. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164-1171
17. Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47-54
18. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236
19. Bruix J and Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022
20. Satake M, Uchida H, Arai Y, Anai H, Sakaguchi H, Nagata T, Yamane T, Kichikawa K, Osaki Y, Okazaki M, Higashihara H, Nakamura H, Osuga K, Nakao N, Hirota S. Transcatheter arterial chemoembolization (TACE) with lipiodol to treat hepatocellular carcinoma: survey results from the TACE study group of Japan. Cardiovasc Intervent Radiol. 2008;31(4):756-761
21. Yau T, Yao TJ, Chan P, Epstein RJ, Ng KK, Chok SH, Cheung TT, Fan ST, Poon RT. The outcomes of elderly patients with hepatocellular carcinoma treated with transarterial chemoembolization. Cancer. 2009;115(23):5507-5515
22. Mondazzi L, Bottelli R, Brambilla G, Rampoldi A, Rezakovic I, Zavaglia C, Alberti A, Idèo G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19(5):1115-1123
23. Poon RT, Fan ST, Lo CM, Liu CL, Ngan H, Ng IO, Wong J. Hepatocellular carcinoma in the elderly: results of surgical and nonsurgical management. Am J Gastroenterol. 1999;94(9):2460-2466
24. Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, Maraldi F, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Farinati F, Zoli M, Borzio F, Giannini EG, Caturelli E, Bernardi M, Trevisani F; Italian Liver Cancer Group. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59(3):387-396
25. Cohen MJ, Bloom AI, Barak O, Klimov A, Nesher T, Shouval D, Levi I, Shibolet O. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19(16):2521-2528
26. Cohen MJ, Levy I, Barak O, Bloom AI, Fernández-Ruiz M, Di Maio M, Perrone F, Poon RT, Shouval D, Yau T, Shibolet O. Trans-arterial chemo-embolization is safe and effective for elderly advanced hepatocellular carcinoma patients: results from an international database. Liver Int. 2014 [Epub ahead of print]
27. Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Sakamoto A, Henmi S, Matsuda F, Eso Y, Ishikawa T, Saito S, Kita R, Kimura T, Osaki Y. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: a proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol. 2011;46(12):1418-1426
28. Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225(1):143-149
29. Nishikawa H, Osaki Y, Kita R, Kimura T, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Saito S, Nasu A. Transcatheter arterial infusion chemotherapy prior to radiofrequency thermal ablation for single hepatocellular carcinoma reduces the risk of intrahepatic distant recurrence. Int J Oncol. 2012;41(3):903-909
30. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Effect of transcatheter arterial chemoembolization prior to surgical resection for hepatocellular carcinoma. Int J Oncol. 2013;42(1):151-160
31. Kudo M, Kubo S, Takayasu K. et al. Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol Res. 2010;40(7):686-692
32. Imaeda T, Yamawaki Y, Seki M, Goto H, Iinuma G, Kanematsu M, Mochizuki R, Doi H, Saji S, Shimokawa K. Lipiodol retention and massive necrosis after lipiodol-chemoembolization of hepatocellular carcinoma: correlation between computed tomography and histopathology. Cardiovasc Intervent Radiol. 1993;16(4):209-213
33. Yamanaka K, Hatano E, Kitamura K, Iida T, Ishii T, Machimito T, Taura K, Yasuchika K, Isoda H, Shibata T, Uemoto S. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol. 2012;47(3):343-346
34. Nishikawa H, Osaki Y, Kita R, Kimura T. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in Japan. Cancers. 2012;4:165-183
35. Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, Baker T, Abecassis MM, Atassi R, Riaz A, Cella D, Burns JL, Ganger D, Benson AB 3rd, Mulcahy MF, Kulik L, Lewandowski R. Increased Quality of Life Among Hepatocellular Carcinoma Patients Treated With Radioembolization, Compared With Chemoembolization. Clin Gastroenterol Hepatol. 2013 [Epub ahead of print]
36. Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 42 Suppl. 2010;3:S206-S214
37. Nishikawa H, Osaki Y, Iguchi E, Takeda H, Ohara Y, Sakamoto A, Hatamaru K, Henmi S, Saito S, Nasu A, Kita R, Kimura T. Percutaneous radiofrequency ablation for hepatocellular carcinoma: clinical outcome and safety in elderly patients. J Gastrointestin Liver Dis. 2012;21(4):397-405
38. Takahashi H, Mizuta T, Kawazoe S, Eguchi Y, Kawaguchi Y, Otuka T, Oeda S, Ario K, Iwane S, Akiyama T, Ozaki I, Fujimoto K. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res. 2010;40(10):997-1005
39. Kao WY, Chiou YY, Hung HH, Su CW, Chou YH, Huo TI, Huang YH, Wu WC, Lin HC, Lee SD, Wu JC. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. J Clin Gastroenterol. 2012;46(1):62-70
40. Kondo K, Chijiiwa K, Funagayama M, Kai M, Otani K, Ohuchida J. Hepatic resection is justified for elderly patients with hepatocellular carcinoma. World J Surg. 2008;32(10):2223-2229
41. Huang J, Li BK, Chen GH, Li JQ, Zhang YQ, Li GH, Yuan YF. Long-term outcomes and prognostic factors of elderly patients with hepatocellular carcinoma undergoing hepatectomy. J Gastrointest Surg. 2009;13(9):1627-1635
42. Su CW, Lei HJ, Chau GY, Hung HH, Wu JC, Hsia CY, Lui WY, Su YH, Wu CW, Lee SD. The effect of age on the long-term prognosis of patients with hepatocellular carcinoma after resection surgery: a propensity score matching analysis. Arch Surg. 2012;147(2):137-144
43. Yeh CN, Lee WC, Jeng LB, Chen MF. Hepatic resection for hepatocellular carcinoma in elderly patients. Hepatogastroenterology. 2004;51(55):219-223
44. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Surgical resection for hepatocellular carcinoma: clinical outcomes and safety in elderly patients. Eur J Gastroenterol Hepatol. 2013;25(8):912-919
45. Zhou L, Rui JA, Wang SB, Chen SG, Qu Q, Chi TY, Wei X, Han K, Zhang N, Zhao HT. Clinicopathological features, post-surgical survival and prognostic indicators of elderly patients with hepatocellular carcinoma. Eur J Surg Oncol. 2006;32(7):767-772
46. Reddy SK, Barbas AS, Turley RS, Gamblin TC, Geller DA, Marsh JW, Tsung A, Clary BM, Lagoo-Deenadayalan S. Major liver resection in elderly patients: a multi-institutional analysis. J Am Coll Surg. 2011;212(5):787-795
47. Takenaka K, Shimada M, Higashi H, Adachi E, Nishizaki T, Yanaga K, Matsumata T, Ikeda T, Sugimachi K. Liver resection for hepatocellular carcinoma in the elderly. Arch Surg. 1994;129(8):846-850
48. Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S, Ichikawa T, Ishikawa H, Nakao K, Eguchi K. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-3043
49. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820-1832
50. Nishikawa H, Osaki Y. Non-B, non-C hepatocellular carcinoma (Review). Int J Oncol. 2013 [Epub ahead of print]
Author contact
![]() Corresponding author: Hiroki Nishikawa, MD. Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan. Tel: +81-6-6774-5111; Fax: +81-6-6774-5131 E-mail: h-nishikawajrc.or.jp.
Corresponding author: Hiroki Nishikawa, MD. Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, 5-30 Fudegasaki-cho, Tennoji-ku, Osaka 543-0027, Japan. Tel: +81-6-6774-5111; Fax: +81-6-6774-5131 E-mail: h-nishikawajrc.or.jp.
Received 2014-4-16
Accepted 2014-6-11
Published 2014-7-17

 Global reach, higher impact
Global reach, higher impact