Abstract
Background:
The phase 3 JAVELIN Renal 101 trial (NCT02684006) demonstrated significantly improved progression-free survival (PFS) with first-line avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma (aRCC). We report updated efficacy data from the second interim analysis.
Patients and methods:
Treatment-naive patients with aRCC were randomized (1 : 1) to receive avelumab (10 mg/kg) intravenously every 2 weeks plus axitinib (5 mg) orally twice daily or sunitinib (50 mg) orally once daily for 4 weeks (6-week cycle). The two independent primary end points were PFS and overall survival (OS) among patients with programmed death ligand 1–positive (PD-L1+) tumors. Key secondary end points were OS and PFS in the overall population.
Results:
Of 886 patients, 442 were randomized to the avelumab plus axitinib arm and 444 to the sunitinib arm; 270 and 290 had PD-L1+ tumors, respectively. After a minimum follow-up of 13 months (data cut-off 28 January 2019), PFS was significantly longer in the avelumab plus axitinib arm than in the sunitinib arm {PD-L1+ population: hazard ratio (HR) 0.62 [95% confidence interval (CI) 0.490–0.777]}; one-sided P < 0.0001; median 13.8 (95% CI 10.1–20.7) versus 7.0 months (95% CI 5.7–9.6); overall population: HR 0.69 (95% CI 0.574–0.825); one-sided P < 0.0001; median 13.3 (95% CI 11.1–15.3) versus 8.0 months (95% CI 6.7–9.8)]. OS data were immature [PD-L1+ population: HR 0.828 (95% CI 0.596–1.151); one-sided P = 0.1301; overall population: HR 0.796 (95% CI 0.616–1.027); one-sided P = 0.0392].
Conclusion:
Among patients with previously untreated aRCC, treatment with avelumab plus axitinib continued to result in a statistically significant improvement in PFS versus sunitinib; OS data were still immature.
Clinical Trial number:
Keywords: avelumab, axitinib, immune checkpoint inhibitor, PD-L1, phase 3, renal cell carcinoma
INTRODUCTION
Antiangiogenic drugs targeting the vascular endothelial growth factor receptor vascular endothelial growth factor receptor (VEGFR) pathway are effective in treating advanced renal cell carcinoma (aRCC).1,2 Axitinib, a VEGFR tyrosine kinase inhibitor, is approved for second-line treatment of aRCC.3,4 Avelumab, a human anti-programmed death ligand 1 (PD-L1) immunoglobulin G1 monoclonal antibody, has shown single-agent activity in aRCC.5
At the first interim analysis of the phase 3 JAVELIN Renal 101 trial (minimum follow-up: 6 months), avelumab plus axitinib demonstrated significantly longer progression-free survival (PFS) than sunitinib in patients with PD-L1+ tumors {hazard ratio (HR) 0.61 [95% confidence interval (CI) 0.47–0.79]; P < 0.001} and in the overall population [HR 0.69 (95% CI 0.56–0.84); P < 0.001]. Overall survival (OS) data were immature at the time.6 Based on these data, the US Food and Drug Administration and the European Commission approved the combination for first-line treatment of aRCC.
We report updated PFS results at the preplanned second interim analysis after a minimum follow-up of 13 months in all patients (data cut-off: 28 January 2019). In addition, updated OS, PFS on next-line therapy (PFS2), mean duration of response (DR), and a rank-preserving structural failure time (RPSFT) analysis of OS that accounts for the subsequent use of anti-PD-1 or anti-PD-L1 inhibitors after progression are reported.
METHODS
Study design and participants
Full trial details were previously described.6 In brief, eligible adults had previously untreated aRCC with a clear-cell component, ≥1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1.
This multicenter, randomized, phase 3 trial was conducted in accordance with ethics principles of the Declaration of Helsinki and the Good Clinical Practice guidelines, defined by the International Council for Harmonisation. All participating patients provided written informed consent.
Outcomes
The two independent primary end points were PFS per RECIST version 1.1 according to blinded independent central review (BICR) and OS in patients with PD-L1+ tumors (≥1% of immune cells staining positive within the tumor area of the tested tissue sample). Key secondary end points were PFS per RECIST version 1.1 according to BICR and OS in the overall population. Other secondary end points included PFS per RECIST version 1.1 according to investigator assessment, objective response (OR) and DR per RECIST version 1.1 according to BICR and investigator assessments, and PFS2 per RECIST version 1.1 according to investigator assessment. Subgroup analyses were prespecified in the statistical analysis plan.
Statistical analysis
Details of the statistical analyses were previously described.6 Additional details are in the supplementary Methods, available at Annals of Oncology online. The second preplanned interim analysis was based on a data cut-off time point when approximately 336 PFS events by BICR occurred in patients with PD-L1+ tumors and the last randomized patient had been followed for ≥12 months after randomization (primary analysis for PFS and second interim analysis for OS). All data reported here are based on the second interim analysis.
Efficacy end points were assessed in all patients who underwent randomization. The OR rate (ORR) was calculated according to treatment with corresponding exact two-sided 95% CIs using the Clopper–Pearson method. PFS, OS, and DR were estimated using the Kaplan–Meier method, and one-sided P values are reported.
PFS2 (defined as the time from randomization to discontinuation of next-line treatment after first objective disease progression by investigator assessment, second objective disease progression by investigator assessment after initiation of next-line treatment, or death from any cause, whichever occurred first) was investigated to determine whether benefit of treatment in the first-line setting had an impact on the benefit of second-line treatment and was used to help understand the relevance of meaningful improvement in PFS. PFS2 was summarized by treatment arm using Kaplan–Meier methodology and displayed graphically. Censoring reasons and hierarchy are shown in the supplementary Table S1, available at Annals of Oncology online.
Ad hoc analyses of mean DR were performed in all randomized patients (irrespective of whether the patient achieved an OR) to enable valid statistical comparison between the two arms.7 This method estimates the mean DR in a timeframe where Kaplan–Meier curves for time to response, progression, or death are well defined. For each randomized patient in the study, DR can be defined as PFS time minus event-free time, where event is subsequently confirmed OR, progressive disease, or death, whichever is earlier. For responders, this corresponds to the DR definition used in conventional responder analyses. For non-responders, this corresponds to a DR of zero. The mean value up to a maximum cut-off follow-up time is equal to the area between the Kaplan–Meier curve of PFS and the Kaplan–Meier curve of event-free time. The mean DR can then be interpreted in a clinically meaningful way as the expected DR for a randomized patient assigned to a treatment.7 This method also addresses the ‘dependent-censoring’ issue associated with the Kaplan–Meier approach for DR (as both censoring and event time depend on time to response).8
The RPSFT model was used to adjust for the subsequent use of PD-1 or PD-L1 inhibitors for patients who previously did not receive avelumab in the trial. Recensoring was implemented to obtain an unbiased estimate of the treatment effect.9,10 Adjusted OS data were assessed using the Cox proportional hazard model, stratified according to the prespecified stratification variables.
Role of the funding source
The trial was sponsored by Pfizer and is part of an alliance between Pfizer and Merck KGaA, Darmstadt, Germany; both companies provided the trial drugs and worked with investigators to design the study; collect, analyze, and interpret the data; and prepare the manuscript. All authors had full access to all data in the study and contributed to the writing, review, and submission of the manuscript.
RESULTS
Between 29 March 2016 and 19 December 2017, 886 patients were randomly assigned to either the avelumab plus axitinib arm (N = 442) or the sunitinib arm (N = 444), and a total of 560 (63.2%) patients had PD-L1+ tumors (N = 270 in the combination arm and N = 290 in the sunitinib arm; supplementary Figure S1, available at Annals of Oncology online). Baseline demographics were previously reported and were balanced between arms.6 As of data cut-off for the second interim analysis (28 January 2019), 242 patients (54.8%) had discontinued both avelumab and axitinib and 336 patients (75.7%) had discontinued sunitinib. Disease progression was the main reason for treatment discontinuation. A total of 170 patients (38.5%) continued to receive avelumab plus axitinib, 8 (1.8%) continued to receive avelumab alone, 22 (5.0%) continued to receive axitinib alone, and 108 (24.3%) continued to receive sunitinib alone.
Among patients in the PD-L1+ population, PFS was significantly longer in the combination arm than in the sunitinib arm [HR 0.62 (95% CI 0.490–0.777); one-sided P < 0.0001; Figure 1A]. The results in the overall population were consistent with those of the PD-L1+ population, demonstrating significantly prolonged PFS in the combination arm versus the sunitinib arm [HR 0.69 (95% CI 0.574–0.825); one-sided P < 0.0001; Figure 1B].
Figure 1. PFS and OS in the PD-L1D population (A and C) and the overall population (B and D).
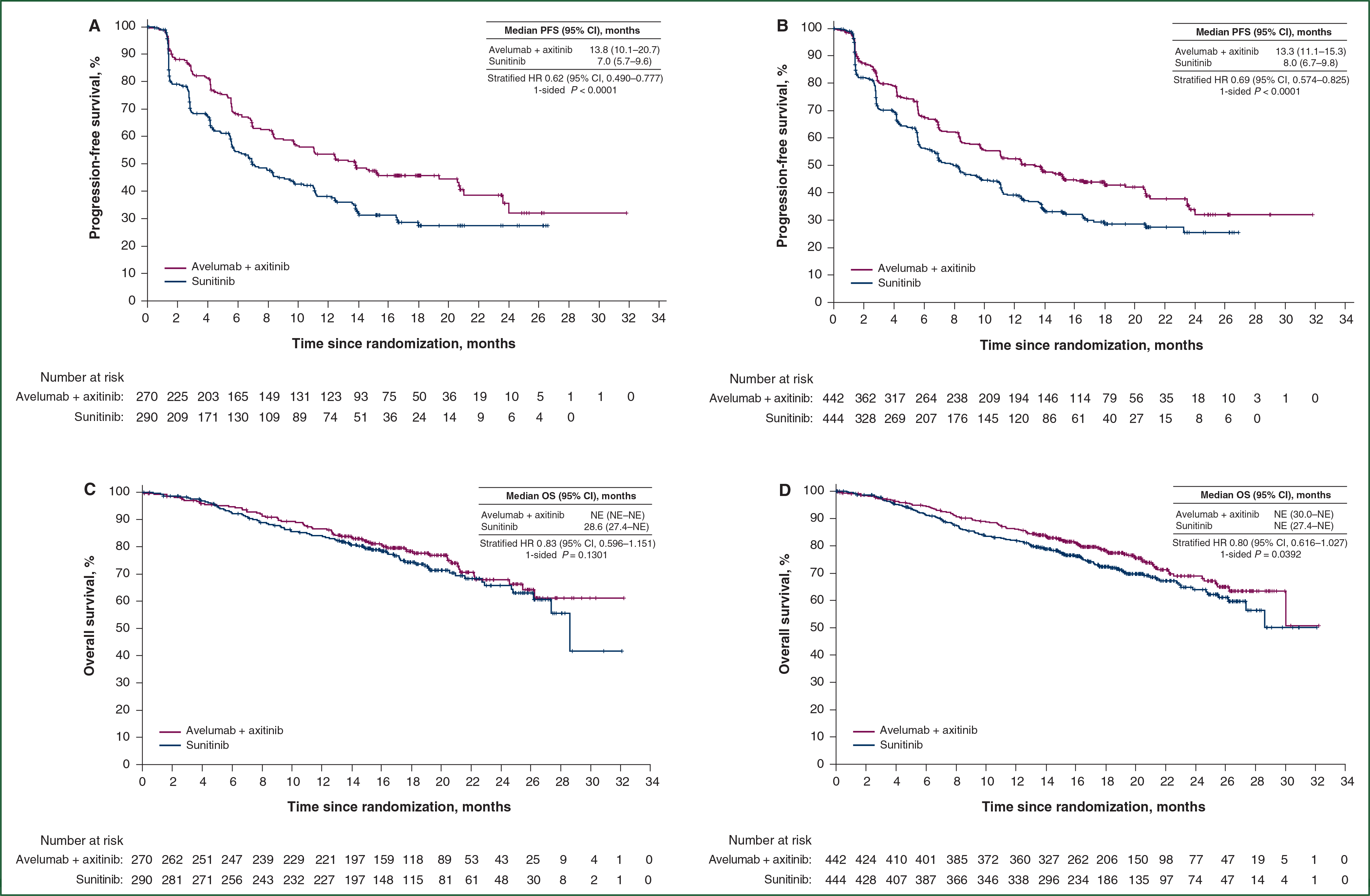
Kaplan–Meier estimates of PFS in the (A) PD-L1+ and (B) overall populations. Progression was defined according to Response Evaluation Criteria in Solid Tumors, version 1.1. Kaplan–Meier estimates of OS in the (C) PD-L1+ and (D) overall populations at the second interim analysis. The OS data were immature. CI, confidence interval; HR, hazard ratio; NE, not estimable; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival.
OS data were still immature at the time of the second interim analysis. Among patients in the PD-L1+ population, the HR was 0.83 (95% CI 0.596–1.151; one-sided P = 0.1301; Figure 1C). Death from any cause was observed in 66 patients (24.4%) in the combination arm and 79 patients (27.2%) in the sunitinib arm. In the overall population, the HR was 0.80 (95% CI 0.616–1.027; one-sided P = 0.0392; Figure 1D). The median duration of follow-up for OS was 19.3 months (95% CI 18.6–20.0) in the combination arm and 19.2 months (95% CI 18.3–19.8) in the sunitinib arm. Deaths from any cause were observed in 109 patients (24.7%) in the combination arm and 129 patients (29.1%) in the sunitinib arm.
Fewer patients in the combination arm than in the sunitinib arm received subsequent anticancer therapy; 138 patients (31.2%) compared with 227 patients (51.1%), respectively (Table 1; supplementary Table S2, available at Annals of Oncology online). A total of 33 patients (7.5%) in the combination arm were treated with any PD-1 or PD-L1 inhibitor compared with 159 patients (35.8%) in the sunitinib arm. Based on the exploratory RPSFT analysis to adjust for subsequent use of any PD-1 or PD-L1 inhibitor in the sunitinib arm, a 35% reduction in the rate of death would have been expected in the overall population [HR 0.65 (bootstrap 95% CI 0.413–0.933)] and PD-L1+ population [HR 0.65 (95% CI 0.337–1.050); Table 1; supplementary Figures S2 and S3, available at Annals of Oncology online]. This predicted result adjusted for the confounding effect of subsequent immuno-oncology therapy.
Table 1.
Subsequent anticancer therapy and adjusted OS in the overall population
| Category | Avelumab plus axitinib (N = 442) | Sunitinib (N = 444) |
|---|---|---|
|
| ||
| Patients with any follow-up anticancer treatments, n (%)a | 138 (31.2) | 227 (51.1) |
| Any VEGF or VEGFR inhibitor | 118 (26.7) | 123 (27.7) |
| Any other drug therapy | 46 (10.4) | 68 (15.3) |
| Any PD-1 or PD-L1 inhibitor | 33 (7.5) | 159 (35.8) |
| Primary OS analysis | ||
| Patients with event, n (%) | 109 (24.7) | 129 (29.1) |
| Stratified analysis | ||
| Hazard ratio (95% CI) | 0.80 (0.616–1.027) | |
| Adjusted OS analysis | ||
| RPSFT analysis | ||
| Hazard ratio (bootstrap 95% CI) | 0.65 (0.413–0.933) | |
CI, confidence interval; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; RPSFT, rank-preserving structural failure time; VEGF(R), vascular endothelial growth factor (receptor).
Patients were counted only once within a given category but may have been counted in more than one category; the denominator to calculate percentages was the number of patients in the full-analysis set.
In the overall population, the confirmed ORR was 52.5% (95% CI 47.7–57.2) with a complete response rate of 3.8% in the combination arm versus an ORR of 27.3% (95% CI 23.2–31.6) and a complete response rate of 2.0% in the sunitinib arm (Table 2; supplementary Figures S4 and S5, available at Annals of Oncology online). The results in the PD-L1+ population were similar to those of the overall population (Table 2; supplementary Figures S6 and S7, available at Annals of Oncology online).
Table 2.
Antitumor activity among PD-L1+ population and overall population
| PD-L1+ population |
Overall population |
|||
|---|---|---|---|---|
| Avelumab plus axitinib (N = 270) | Sunitinib (N = 290) | Avelumab plus axitinib (N = 442) | Sunitinib (N = 444) | |
|
| ||||
| Confirmed objective response rate (95% CI), % | 55.9 (49.8–61.9) | 27.2 (22.2–32.8) | 52.5 (47.7–57.2) | 27.3 (23.2–31.6) |
| Confirmed best overall response, n (%) | ||||
| Complete response | 15 (5.6) | 7 (2.4) | 17 (3.8) | 9 (2.0) |
| Partial response | 136 (50.4) | 72 (24.8) | 215 (48.6) | 112 (25.2) |
| Stable disease | 73 (27.0) | 120 (41.4) | 125 (28.3) | 194 (43.7) |
| Progressive disease | 31 (11.5) | 65 (22.4) | 55 (12.4) | 86 (19.4) |
| Not evaluable | 11 (4.1)a | 20 (6.9)b | 24 (5.4)c | 34 (7.7)d |
| Othere | 4 (1.5) | 6 (2.1) | 6 (1.4) | 9 (2.0) |
| Patients with ongoing response, n/N (%) | 84/151 (55.6) | 42/79 (53.2) | 126/232 (54.3) | 61/121 (50.4) |
CI, confidence interval; NE, not estimable; PD-L1, programmed death ligand 1.
No postbaseline assessments due to early death or other reasons (n = 9); stable disease <6 weeks after randomization (n = 2).
Stable disease <6 weeks after randomization (n = 9); no postbaseline assessments due to early death or other reasons (n = 8); new anticancer therapy started before first postbaseline assessment (n = 2); all postbaseline assessments have overall response of not evaluable (n = 1).
No postbaseline assessments due to early death or other reasons (n = 17); stable disease <6 weeks after randomization (n = 5); no adequate baseline assessment (n = 2).
Stable disease <6 weeks after randomization (n = 15); no postbaseline assessments due to early death or other reasons (n = 13); new anticancer therapy started before first postbaseline assessment (n = 3); no adequate baseline assessment (n = 2); all postbaseline assessments have overall response of not evaluable (n = 1).
Patients without target lesions at baseline per independent review who achieved noncomplete response/nonprogressive disease.
In the overall population, responders in the combination arm had an earlier onset of response than those in the sunitinib arm [median time to response was 2.7 months (range 1.2–20.7) in the combination arm versus 4.0 months (range 1.2–18.0) in the sunitinib arm]. The same trend was observed in the PD-L1+ population [median time to response was 2.0 months (range 1.2–20.7) versus 3.1 months (range, 1.2–12.5), respectively].
Responses in both treatment arms were durable. In the overall population, the median DR was 18.5 months (95% CI 17.8 to not estimable) in the combination arm and not estimable (95% CI 16.4 to not estimable) in the sunitinib arm. In the PD-L1+ population, the median DR was 18.5 months (95% CI 17.8 to not estimable) in the combination arm and not estimable (95% CI 11.2 to not estimable) in the sunitinib arm. Mean DR was analyzed in all randomized patients for valid statistical comparison between the two treatment arms,7 and the analysis showed the mean DR was 4.2 months longer (95% CI 2.9–5.6) in the combination arm than the sunitinib arm (Figure 2A). Similarly, in the PD-L1+ population, the mean DR was 4.7 months longer (95% CI 3.1–6.3) in the combination arm than the sunitinib arm (supplementary Figure S8, available at Annals of Oncology online).
Figure 2. Mean (A) DR and (B) PFS2 in the overall population.
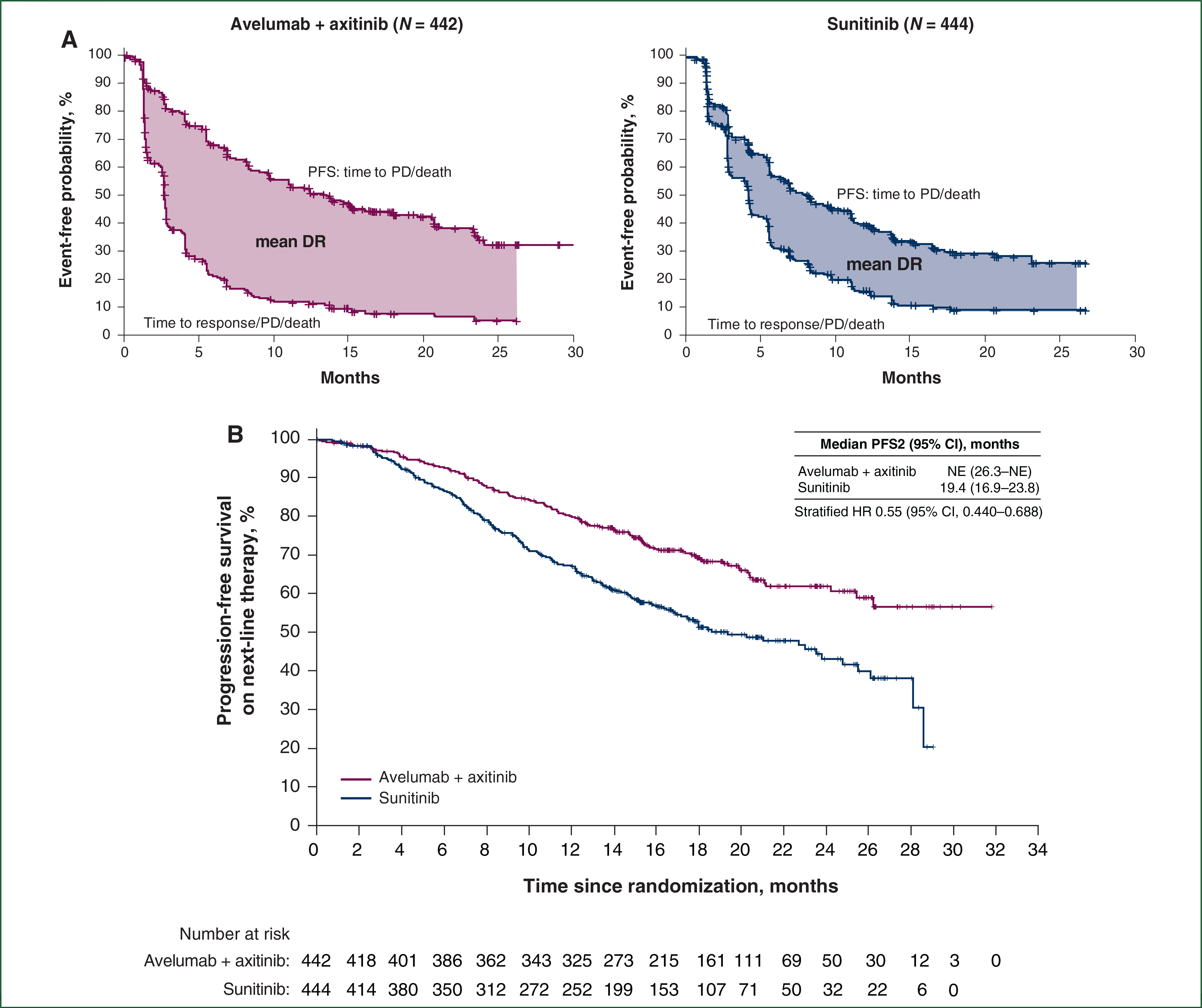
(A) Kaplan–Meier estimates of PFS (upper curves) and time to response/progressive disease (PD)/death (lower curves) for avelumab plus axitinib and sunitinib in the overall population. The difference in mean DR was 4.2 months (95% CI 2.9–5.6), and the truncation time τ was 26.25 months. DR is equal to PFS time minus time to response, PD, or death (whichever is earliest). (B) Kaplan–Meier estimate of PFS on next-line therapy (PFS2) for avelumab plus axitinib and sunitinib in the overall population. CI, confidence interval; DR, duration of response; HR, hazard ratio; NE, not estimable; PFS, progression-free survival.
To investigate whether first-line treatment impacted the benefit of second-line treatment, PFS2 was analyzed. In the overall population, the HR was 0.55 (95% CI 0.440–0.688), favoring the combination arm (Figure 2B). PFS2 results in the PD-L1+ population were similar to those of the overall population [HR 0.52 (95% CI 0.395–0.694); supplementary Figure S9, available at Annals of Oncology online].
Overall, PFS and OS results favored avelumab plus axitinib over sunitinib across prespecified subgroups, including ECOG PS score, PD-L1 status, and prognostic risk groups in both the overall (Figure 3A and B) and PD-L1+ populations (supplementary Figures S10 and S11, available at Annals of Oncology online). In addition, OR results favored the combination in all prespecified subgroups assessed in both the overall and PD-L1+ populations (Figure 3C; supplementary Figure S12, available at Annals of Oncology online).
Figure 3. Subgroup analyses of (A) PFS, (B) OS, and (C) OR in the overall population.

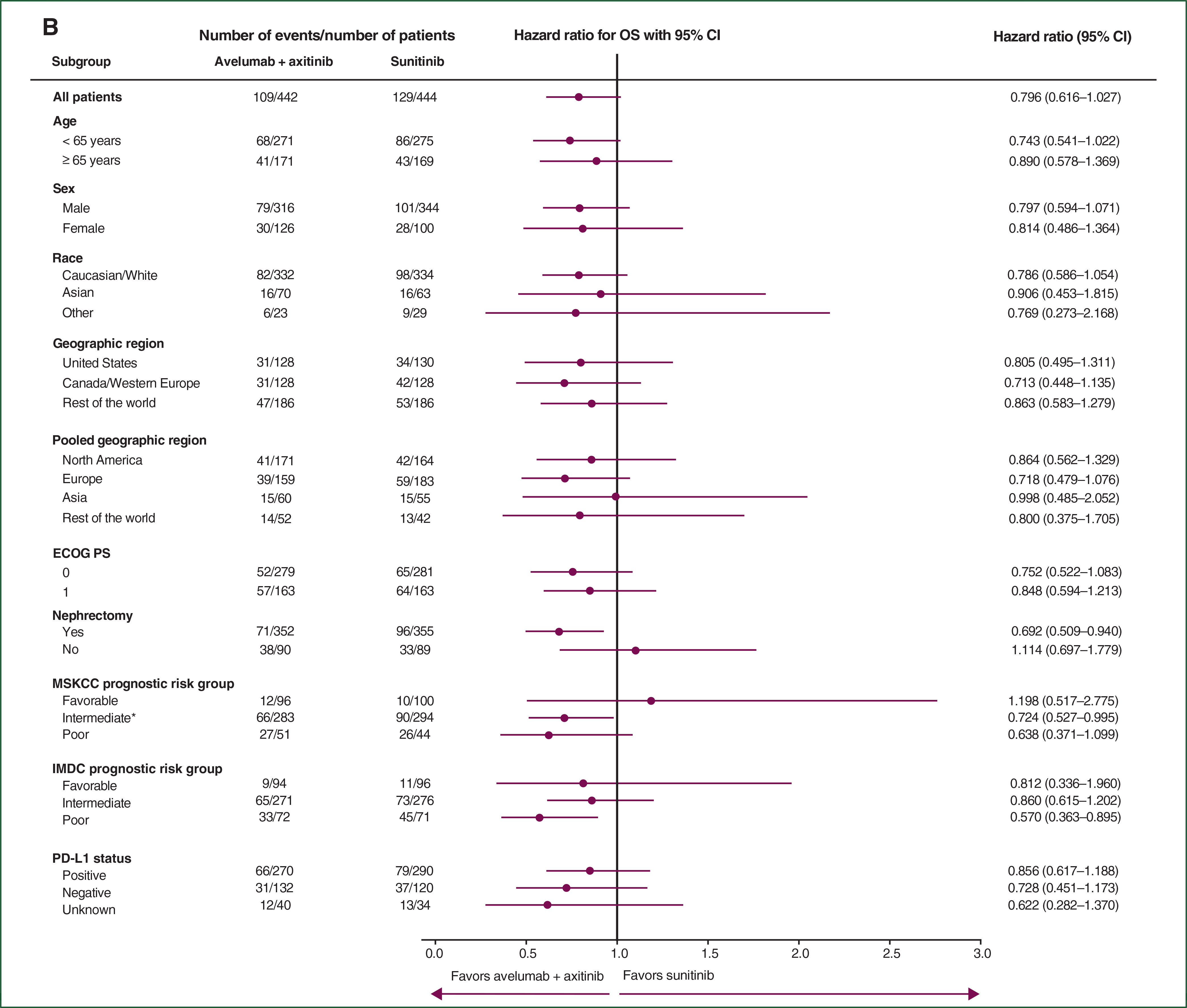

*Compared with the originally reported baseline data,6 one patient in the sunitinib arm, who was initially classified with poor-risk disease, was subsequently reclassified as having intermediate-risk disease for this analysis due to a correction in a normal range used for a laboratory value. BICR, blinded independent central review; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; MSKCC, Memorial Sloan Kettering Cancer Center; OR(R), objective response (rate); OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival.
PFS, OS, and OR by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic risk groups are shown in Table 3. Among patients in the overall population with favorable-, intermediate-, and poor-risk disease, the HR for PFS and OS favored the combination arm over the sunitinib arm. Furthermore, an OR was achieved in a higher proportion of patients in the combination arm than in the sunitinib arm across all risk groups.
Table 3.
Efficacy outcomes in IMDC risk groups in the overall population
| IMDC risk group | PFS, median (95% CI), months |
OS, median (95% CI), months |
ORR (95% CI), % |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Avelumab plus axitinib (N = 442) | Sunitinib (N = 444) | HR (95% CI) | Avelumab plus axitinib (N = 442) | Sunitinib (N = 444) | HR (95% CI) | Avelumab plus axitinib (N = 442) | Sunitinib (N = 444) | Odds ratio (95% CI) | |
|
| |||||||||
| Favorable | 24.0 (20.7–NE) | 16.7 (12.6–NE) | 0.626 (0.397–0.986) | NE (NE) | NE (NE) | 0.812 (0.336–1.960) | 67.0 (56.56–76.38) | 39.6 (29.75–50.08) | 3.102 (1.645–5.869) |
| Intermediate | 11.6 (8.4–15.2) | 8.3 (6.9–11.0) | 0.756 (0.603–0.948) | 30.0 (30.0–NE) | 28.6 (27.4–NE) | 0.860 (0.615–1.202) | 53.1 (47.00–59.20) | 26.8 (21.68–32.45) | 3.095 (2.132–4.500) |
| Poor | 6.0 (3.0–9.0) | 2.9 (2.7–5.6) | 0.514 (0.342–0.774) | 21.2 (14.7–26.3) | 11.0 (7.8–16.5) | 0.570 (0.363–0.895) | 31.9 (21.44–43.99) | 12.7 (5.96–22.70) | 3.234 (1.288–8.627) |
CI, confidence interval; HR, hazard ratio; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; NE, not estimable; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Safety assessment was continued according to the protocol, and there were no new safety concerns.
DISCUSSION
In this final analysis of the primary PFS end point (by BICR), avelumab plus axitinib continued to show a statistically significant benefit compared with sunitinib in prolonging PFS in the first-line treatment of patients with aRCC. The magnitude of benefit was consistent with that observed at the time of the first interim analysis,6 with a 38% rate reduction and a 6.8-month longer median PFS in the PD-L1+ population and a 31% rate reduction and a 5.3-month longer median PFS in the overall population. An OR benefit was also observed for all patients in the combination arm regardless of PD-L1 expression. In addition, across prespecified subgroups, including prognostic risk groups, PFS and OR consistently favored the combination over sunitinib. In an exploratory analysis, the combination had a longer mean DR than sunitinib for all randomized patients and in the PD-L1+ population. The combination also prolonged PFS2 compared with sunitinib in the overall population and the PD-L1+ population, suggesting that there is no negative impact of first-line treatment with the combination on subsequent benefit from second-line treatment.
The OS data were still immature, with a median follow-up for OS of ~19 months and 27% deaths observed across both arms in the overall population. Although no definitive conclusions can be drawn and OS data continue to be evaluated in this trial, several lines of evidence collectively suggest a benefit for avelumab in combination with axitinib in prolonging OS time compared with sunitinib: the point estimates in the PD-L1+ [HR 0.828 (95% CI 0.596–1.151)] and overall [HR 0.796 (95% CI 0.616–1.027)] populations and in subgroups of patients with poor prognosis among the overall population [IMDC: HR 0.570 (95% CI 0.363–0.895]; MSKCC: HR 0.638 (95% CI 0.371–1.099)] favor the combination over sunitinib, and a further reduction in the rate of death for the combination arm was predicted [HR 0.65 (bootstrap 95% CI 0.413–0.933)] after correction for the confounding effect of subsequent PD-1 or PD-L1 inhibitor therapies using the RPSFT method.
Continued assessment of safety in this trial did not identify any new safety concerns, and the safety profile of the combination was consistent with those of avelumab and axitinib when administered as monotherapy or in combination.3,6,11–13
Other phase 3 trials assessing combination therapies in the first-line treatment of aRCC have recently been reported. The CheckMate 214 trial assessed nivolumab (anti-PD-1) plus ipilimumab (anti-CTLA-4) as treatment for patients with intermediate or poor prognostic risk.14 At a median follow-up of 32.4 months, there was a significant survival benefit for the combination versus sunitinib (HR 0.66; P < 0.0001). PFS was significantly longer with the combination versus sunitinib (HR 0.77; P = 0.0014), and a significantly higher proportion of patients achieved an OR (42% versus 29%; P = 0.0001).14,15 The IMmotion151 trial assessed atezolizumab (anti-PD-L1) plus bevacizumab (anti-VEGF) versus sunitinib.16 In the intention-to-treat population, with median follow-up of 24 months, median OS was 33.6 months in the combination arm versus 34.9 months in the sunitinib arm, and the results (HR 0.93) had not yet crossed the significance boundary. Median PFS was 11.2 months in the combination arm versus 8.4 months in the sunitinib arm (HR 0.83; P = 0.0219). An OR was achieved in 37% of patients in the atezolizumab plus bevacizumab arm versus 33% in the sunitinib arm.16 The KEYNOTE-426 trial assessed pembrolizumab (anti-PD-1) plus axitinib versus sunitinib.17,18 With a median follow-up of 16.6 months for survival, OS and PFS were significantly longer in the combination arm versus the sunitinib arm [HR 0.59 (P = 0.0001) and HR 0.69 (P < 0.0001), respectively].18 The ORR was 59.3% in the combination arm and 35.7% in the sunitinib arm (P < 0.001; median follow-up of 12.8 months).17 Although there are important differences in key design elements that make cross-trial comparisons difficult, such as the proportions of patients among IMDC prognostic risk groups and access to and rate of subsequent therapy use with PD-1/PD-L1 inhibitors, the current study adds to the evidence that dual targeting of multiple pathways is an effective therapeutic strategy for patients with untreated aRCC.
In conclusion, the updated efficacy results were consistent with those previously reported and demonstrated that avelumab in combination with axitinib has a clinically meaningful and statistically significant benefit in prolonging PFS and results in an approximate doubling of ORR compared with sunitinib in first-line treatment of patients with aRCC. This benefit was observed across several subgroups, including all IMDC prognostic risk groups, with important insights based on improvements in PFS2 and mean DR.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers. This trial was supported by Pfizer and is part of an alliance between Pfizer and Merck KGaA, Darmstadt, Germany. The conduct of the trial at the Memorial Sloan Kettering Cancer Center was supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). Medical writing support was provided by Kelly Bryant, ClinicalThinking (Hamilton, NJ, USA), and funded by Pfizer and Merck KGaA, Darmstadt, Germany.
FUNDING
Pfizer and Merck KGaA, Darmstadt, Germany (no grant numbers).
DISCLOSURE
TKC reports the following: Research (institutional and personal): AstraZeneca, Alexion, Bayer, Bristol-Myers Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, Takeda. Honoraria: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol-Myers Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OncLive, PeerView, and PER), Research to Practice, Lpath, Kidney Cancer Journal, Clinical Care Options, PlatformQ, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, New England Journal of Medicine, The Lancet Oncology, Heron Therapeutics, Lilly. Consulting or Advisory Role: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol-Myers Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, Pionyr, Tempest. Stock ownership: Pionyr, Tempest. Other present or past leadership roles: Director of GU Oncology Division at Dana-Farber and past President of Medical Staff at Dana-Farber, member of NCCN Kidney Panel and the GU Steering Committee, past chairman of the Kidney Cancer Association Medical and Scientific Steering Committee. Patents, royalties or other intellectual properties: International Patent Application No. PCT/US2018/12209, entitled ‘PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response’, filed 3 January 2018, claiming priority to U.S. Provisional Patent Application No. 62/445,094, filed 11 January 2017; International Patent Application No. PCT/US2018/058430, entitled ‘Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy’, filed 31 October 2018, claiming priority to U.S. Provisional Patent Application No. 62/581,175, filed 3 November 2017. Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies (ClinicalThinking, Envision Pharma Group, Fishawack). RJM reports personal fees and other from Pfizer during the conduct of the study; personal fees and other from Genentech/Roche, personal fees from Incyte, other from Bristol-Myers Squibb, personal fees and other from Novartis, personal fees and other from Exelixis, personal fees and other from Eisai, personal fees from Merck, outside the submitted work. BIR reports grants and personal fees from Pfizer during the conduct of the study, grants and personal fees from Merck (MSD), grants and personal fees from Bristol-Myers Squibb, personal fees from Novartis, grants and personal fees from GNE/Roche, personal fees from Exelixis, personal fees from Peloton, outside the submitted work. JH reports grants and personal fees from Bristol-Myers Squibb, MSD, Novartis, and Neon Therapeutics; personal fees from Pfizer, Roche/Genentech, Bayer, Immunocore, Seattle Genetics, Gadeta B.V., Celsius Therapeutics, and AstraZeneca/MedImmune, outside the submitted work. MTC reports personal fees from Eisai, grants and personal fees from EMD Serono, grants from Pfizer, personal fees from Genentech and AstraZeneca, grants from Exelixis and Janssen, other from Bristol-Myers Squibb, Roche, and Merck, outside the submitted work. BV reports personal fees from Bristol-Myers Squibb, personal fees and nonfinancial support from Merck Serono Dohme (MSD), personal fees from EUSA Pharma, personal fees and nonfinancial support from Ipsen, during the conduct of the study. CK reports personal fees from Pfizer, Bristol-Myers Squibb, Eisai, Ipsen, Astellas, and EMD Serono, outside the submitted work. GG-M and MU have nothing to disclose. JLL reports grants, personal fees, and other from Pfizer Korea and Ipsen Korea, personal fees and other from Janssen and Sanofi Aventis, personal fees from Novartis Korea, and personal fees and other from Astellas Korea and Bristol-Myers Squibb Korea, outside the submitted work. M-OG reports grants and personal fees from Novartis and Bristol-Myers Squibb, personal fees from Pfizer, Bayer HealthCare, Astellas, Intuitive Surgical, Sanofi Aventis, Hexal, APOGEPHA, Amgen, AstraZeneca, MSD, Janssen Cilag, Ono Pharma, Ipsen Pharma, Medac, and Merck, outside the submitted work. HG reports personal fees from Bristol-Myers Squibb, Astellas, Pfizer, AstraZeneca, Ipsen, Roche, and MSD, outside the submitted work. MS reports grants, personal fees, and nonfinancial support from Pfizer; personal fees and nonfinancial support from Roche; and personal fees from Novartis, Bristol-Myers Squibb, Ipsen, Exelixis, Eisai, Astellas, and EUSA Pharma, outside the submitted work. JL reports grants and personal fees from Achilles Therapeutics, MSD, Bristol-Myers Squibb, Nektar, Novartis, Pfizer, Roche/Genentech, and Immunocore; personal fees from AstraZeneca, Boston Biomedical, Eisai, EUSA Pharma, GlaxoSmithKline, Ipsen, Imugene, Incyte, iOnctura, Kymab, Merck Serono, Pierre Fabre, Secarna, Vitaccess, and Covance; and grants from Aveo and Pharmacyclics, outside the submitted work. MBA reports personal fees from Pfizer, Bristol-Myers Squibb, Merck, Roche, Exelixis, Eisai, Novartis, Alexion, Boehringer Ingelheim, Nektar, and X4 Pharmaceuticals, outside the submitted work. SKP has received honoraria from Astellas Pharma, Medivation, and Novartis; and fees for a consulting or advisory role from Aveo, Bristol-Myers Squibb, Exelixis, Genentech, Myriad Pharmaceuticals, Novartis, and Pfizer. JW, MM, SK, PC, AC, CF, BH, and AdP are employees of Pfizer. LA reports personal fees from Bristol-Myers Squibb, Pfizer, Ipsen, Peloton Therapeutics, Roche, MSD, and Novartis, outside the submitted work.
DATA SHARING
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
REFERENCES
- 1.Zarrabi K, Fang C, Wu S. New treatment options for metastatic renal cell carcinoma with prior anti-angiogenesis therapy. J Hematol Oncol. 2017;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. [DOI] [PubMed] [Google Scholar]
- 3.Inlyta (Axitinib) [Prescribing Information]. New York, NY: Pfizer; 2018. [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: kidney cancer. Available at https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. Accessed December 10, 2019. [DOI] [PubMed]
- 5.Vaishampayan U, Schoffski P, Ravaud A, et al. Avelumab monotherapy as first-line or second-line treatment in patients with metastatic renal cell carcinoma: phase Ib results from the JAVELIN Solid Tumor trial. J Immunother Cancer. 2019;7:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang B, Tian L, Talukder E, et al. Evaluating treatment effect based on duration of response for a comparative oncology study. JAMA Oncol. 2018;4:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin DY, Sun W, Ying Z. Nonparametric estimation of the gap time distributions for serial events with censored data. Biometrika. 1999;86: 59–70. [Google Scholar]
- 9.Allison A, White IR, Bond S. rpsftm: an R package for rank preserving structural failure time models. R J. 2017;9:342–353. [PMC free article] [PubMed] [Google Scholar]
- 10.Latimer NR, Abrams KR. NICE DSU technical support document 16: adjusting survival time estimates in the presence of treatment switching. Available at http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD16_Treatment_Switching.pdf. Accessed December 10, 2019. [PubMed]
- 11.Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018;124:2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19: 451–460. [DOI] [PubMed] [Google Scholar]
- 13.Bavencio (Avelumab) [Package Insert]. Darmstadt, Germany: Merck KGaA; 2019. [Google Scholar]
- 14.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 18.Keytruda (Pembrolizumab) [Summary of Product Characteristics]. Haarlem, the Netherlands: Merck Sharp & Dohme BV; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


