PURPOSE
We assessed the safety and efficacy of cabozantinib and nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) in patients with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignances.
PATIENTS AND METHODS
Patients received escalating doses of CaboNivo or CaboNivoIpi. The primary objective was to establish a recommended phase II dose (RP2D). Secondary objectives included objective response rate (ORR), progression-free survival (PFS), duration of response (DoR), and overall survival (OS).
RESULTS
Fifty-four patients were enrolled at eight dose levels with a median follow-up time of 44.6 months; data cutoff was January 20, 2020. Grade 3 or 4 treatment-related adverse events (AEs) occurred in 75% and 87% of patients treated with CaboNivo and CaboNivoIpi, respectively, and included fatigue (17% and 10%, respectively), diarrhea (4% and 7%, respectively), and hypertension (21% and 10%, respectively); grade 3 or 4 immune-related AEs included hepatitis (0% and 13%, respectively) and colitis (0% and 7%, respectively). The RP2D was cabozantinib 40 mg/d plus nivolumab 3 mg/kg for CaboNivo and cabozantinib 40 mg/d, nivolumab 3 mg/kg, and ipilimumab 1 mg/kg for CaboNivoIpi. ORR was 30.6% (95% CI, 20.0% to 47.5%) for all patients and 38.5% (95% CI, 13.9% to 68.4%) for patients with mUC. Median DoR was 21.0 months (95% CI, 5.4 to 24.1 months) for all patients and not reached for patients with mUC. Median PFS was 5.1 months (95% CI, 3.5 to 6.9 months) for all patients and 12.8 months (95% CI, 1.8 to 24.1 months) for patients with mUC. Median OS was 12.6 months (95% CI, 6.9 to 18.8 months) for all patients and 25.4 months (95% CI, 5.7 to 41.6 months) for patients with mUC.
CONCLUSION
CaboNivo and CaboNivoIpi demonstrated manageable toxicities with durable responses and encouraging survival in patients with mUC and other GU tumors. Multiple phase II and III trials are ongoing for these combinations.
INTRODUCTION
An estimated 362,860 new genitourinary (GU) tumors are expected to be diagnosed in the United States in 2020.1 Treatment options for these tumors have changed in recent years. The US Food and Drug Administration recently approved seven new agents for metastatic urothelial carcinoma (mUC), including five immune checkpoint inhibitors (ICIs).2-6 In addition, the development of antiangiogenic agents and ICIs for metastatic renal cell carcinoma (mRCC) has led to survival benefits,7 and new androgen receptor and poly(ADP-ribose) polymerase inhibitors have demonstrated clinical benefit in castration-resistant prostate cancer (CRPC).8,9 Yet, in the metastatic setting, these diseases are incurable,7,10 and effective treatment options are still needed, especially for less common GU histologies.
CONTEXT
Key Objective
We aimed to evaluate the safety and clinical activity of cabozantinib in combination with nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) for patients with metastatic genitourinary (GU) tumors.
Knowledge Generated
There were no dose-limiting toxicities with cabozantinib 60 mg daily; however, there were many grade 1 and 2 adverse events (AEs) and dose holdings or reductions. The recommended phase II doses were cabozantinib 40 mg, nivolumab 3 mg/kg, and ipilimumab 1 mg/kg. The most common AEs of any grade were fatigue, diarrhea, and anorexia. The study showed promising clinical activity, with an overall response rate of 30.6%.
Relevance
We demonstrated that the combinations CaboNivo and CaboNivoIpi are feasible and have promising clinical activity. Our results have led to the development of expansion cohorts and larger clinical trials evaluating these combinations in several types of cancer.
Cabozantinib inhibits multiple receptor tyrosine kinases (TKs) involved in tumor growth, angiogenesis, and immune cell regulation, including MET, VEGFR, RET, KIT, TIE-2, ROS1, and the TAM family of kinases (TYRO3, AXL, and MER).11 VEGFR2 contributes to tumor angiogenesis, carcinogenesis, and progression of GU malignancies such as urothelial carcinoma, renal cell carcinoma (RCC), and prostate cancer.12,13 The MET pathway also has an important role in the tumorigenesis of these tumors and seems to cooperate with the VEGF pathway in tumor angiogenesis.13,14 Preclinical models have suggested that the MET pathway mediates resistance to VEGF-targeted therapy in several cancers, including RCC,15,16 and multiple clinical trials investigating cabozantinib in GU tumors have shown clinical activity.17-19
ICIs are now part of the standard of care for mUC and mRCC7,10 and have been investigated in CRPC and metastatic germ cell tumors (mGCTs).20,21 Nivolumab is a monoclonal antibody against the programmed cell death protein 1 (PD-1) cell surface membrane receptor.22 The clinical activity of nivolumab has been reported in clinical trials for patients with mRCC23 and mUC.3,24 Ipilimumab is a monoclonal antibody specific for human cytotoxic T-lymphocyte antigen 4 (CTLA-4).25 The PD-1 and CTLA-4 signaling cascades use nonredundant mechanisms to block T-cell activation,26 and clinically, the combination of ipilimumab and nivolumab has shown meaningful activity in patients with mRCC27 and mUC.28
TK inhibitors (TKIs) against VEGFR and other receptor tyrosine kinases may have antitumor immune-mediated mechanisms. Preclinical studies have shown that antiangiogenic TKIs, such as cabozantinib, can modify the tumor microenvironment by reducing the percentage of immunosuppressive T regulatory cells and myeloid-derived suppressor cells and can increase T-cell infiltration.17,29-31 In addition, the combination of anti-VEGF–targeted therapies with ICIs has shown improvements in clinical outcomes for patients with mRCC32-34 and CRPC.35
The objectives of this phase I trial were to determine dose-limiting toxicities (DLTs) and the recommended phase II dose (RP2D) for the combinations of cabozantinib and nivolumab (CaboNivo) and cabozantinib, nivolumab, and ipilimumab (CaboNivoIpi) in patients with GU tumors and to assess the clinical efficacy of these combinations.
PATIENTS AND METHODS
Patient Selection
Eligible patients had a histologically confirmed diagnosis of metastatic GU tumors with new or progressive lesions on cross-sectional imaging, measurable by RECIST v1.1.36 Patients must have received one or more lines of standard therapy unless no standard treatment existed that had been shown to prolong survival. For complete inclusion and exclusion criteria, see the Data Supplement.
The study protocol (ClinicalTrials.gov identifier: NCT02496208) was approved by institutional review boards at all participating institutions. Patients were enrolled per international standards of good clinical practice and institutional safety monitoring. All patients provided written informed consent before study entry.
Study Design
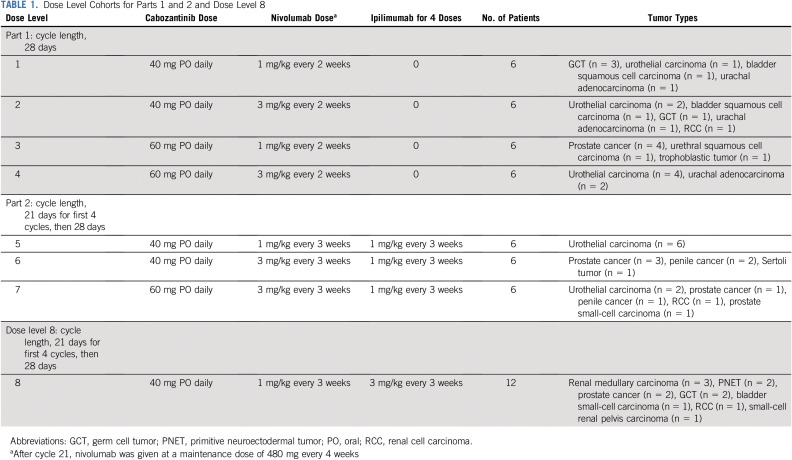
This phase I dose-escalation study initially had seven dose levels divided into two parts (Table 1). The study used a rolling six, phase I trial design.37 Two to six patients could be concurrently enrolled onto a dose level.
TABLE 1.
Dose Level Cohorts for Parts 1 and 2 and Dose Level 8
The DLT period refers to the first 4 weeks for CaboNivo and the first 6 weeks for CaboNivoIpi during the dose-escalation phase for all seven dose levels. A DLT was defined as an adverse event (AE) potentially attributable to any of the study drugs or the combination that required permanent discontinuation of protocol therapy or was grade ≥ 3 according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 5.0. If dose reduction or interruption of cabozantinib led to a patient taking ≤ 75% of the planned dose within the DLT observation period, the event was considered a DLT.
Dose level 8 was added after completion of the dose-escalation portion of the study as an exploratory cohort of 12 patients to assess the safety and efficacy of CaboNivoIpi with a higher dose of ipilimumab (3 mg/kg; Table 1). This cohort was added after the results of the phase I/II CheckMate 032 study were first presented28 suggesting that ipilimumab 3 mg/kg plus nivolumab 1 mg/kg was more active in mUC than ipilimumab 1 mg/kg plus nivolumab 3 mg/kg.
Treatment
Part 1 had four escalating dose levels of continuous daily oral cabozantinib and intravenous (IV) nivolumab administrated every 2 weeks for a 28-day cycle (Table 1). Restaging was performed every 8 weeks.
Part 2 started after part 1 enrollment was completed and had three escalating dose levels of continuous oral daily cabozantinib, with nivolumab and ipilimumab administrated IV every 21 days during the first four cycles and then nivolumab every 14 days thereafter (Table 1). The first four cycles lasted 21 days; subsequent cycles lasted 28 days. Restaging was performed every 6 weeks during the first four cycles while on ipilimumab and then every 8 weeks thereafter.
After cycle 21, nivolumab was given at a maintenance dose of 480 mg every 4 weeks with daily cabozantinib. All patients who achieved a partial response (PR) or complete response (CR) by RECIST criteria had the option of discontinuing therapy 2 years after the PR or CR was confirmed. Patients who had progressive disease (PD) and still met eligibility criteria could enroll on an exploratory ipilimumab challenge cohort (for part 1 patients) or ipilimumab rechallenge cohort (for part 2 patients who achieved stable disease [SD] for 6 months, CR, or PR as best response). Patients could then receive four cycles of CaboNivoIpi every 3 weeks, followed by CaboNivo every 2 weeks and daily cabozantinib at current dose.
Dose reductions for cabozantinib (40 mg/d, 20 mg/d, then 20 mg every other day) and interruptions of study treatment were specified for management of AEs. After dose reduction, no dose escalation was permitted. No dose modification was allowed for ICIs. Patients could discontinue treatment as a result of PD, unacceptable toxicity, or withdrawal of consent or based on the investigator’s clinical judgment. If one drug was discontinued, the patient could remain on the other drug(s). Treatment beyond PD was permitted if the patient tolerated treatment and the investigator considered that the patient would benefit clinically.
Outcomes
The primary objective of this phase I, open-label, dose-escalation trial was to determine DLTs and the RP2D of CaboNivo and CaboNivoIpi in patients with GU tumors. Secondary end points included evaluation of clinical activity of the study combinations, as determined by investigator-assessed confirmed objective response rate (ORR; proportion of patients with a confirmed best response of CR or PR) using RECIST v1.1, disease control rate (DCR; proportion of patients with a confirmed best response of CR, PR, or SD), duration of response (DoR), progression-free survival (PFS), and overall survival (OS). Another secondary end point was the detection and clinical correlation of epithelial cell adhesion molecule (EpCAM)–positive circulating tumor cells (CTCs) with additional markers (MET, CXCR4, and PD-L1) using multiparameter flow cytometry, as previously described.38 The cutoff of < or ≥ 5 CTCs per 10 mL of whole blood was used.
Statistical Analysis
Follow-up was calculated as the median of the potential follow-up intervals for each patient from the on-study date until the date the data were locked (January 20, 2020). Safety and clinical activity (PFS and OS) were analyzed in all patients. The ORR was estimated, along with an exact 95% CI. The 95% CIs were determined using the exact Clopper-Pearson method. DoR was defined as the date the response was noted until date of radiologic PD, clinical PD, or death. PFS and OS were estimated using the Kaplan-Meier method, starting from the on-study date until PD, death, or last follow-up, as appropriate, with PFS being defined as progression or death without prior progression. For responding patients, PFS and OS were determined starting from the date of response until the date of death, PD, or last follow-up. The Kaplan-Meier plots and all analysis were done using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patients with GU tumors (N = 54) were enrolled in this study from July 2015 through August 2017 (CaboNivo, n = 24; CaboNivoIpi, n = 30). Baseline demographics and clinical characteristics are listed in Table 2.
TABLE 2.
Patient Characteristics

Six patients in seven dose levels completed the dose-escalation phase, and 12 patients were treated at dose level 8. All 54 patients were evaluable for safety and time-event outcomes. Five patients (CaboNivo, n = 1; CaboNivoIpi, n = 4) had early PD or withdrew before completing cycle 1 and were not evaluable for ORR.
Median follow-up time was 44.6 months for all patients, the median duration of treatment was 4.8 months (interquartile range [IQR], 2.1-16.3 months), and time to best response was 1.9 months (IQR, 1.7-2.8 months). For patients who received CaboNivo, the median duration of treatment was 6.36 months (IQR, 2.66-19.51 months), and the time to best response was 1.81 months (IQR, 1.71-3.68 months). Patients who received CaboNivoIpi had a median duration of treatment of 3.7 months (IQR, 2.07-7.62 months), and the median time to best response was 1.94 months (IQR, 1.71-2.79 months).
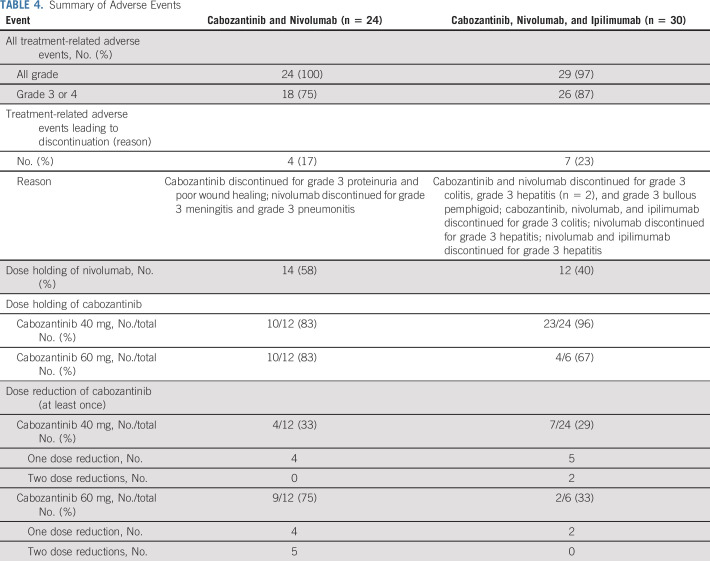
The most common treatment-related AEs (TRAEs) of any grade and grade 3 or 4 per cabozantinib dose and the most common reasons for treatment discontinuation, dose hold, and dose reduction are reported in Tables 3 and 4 and the Data Supplement. No DLTs were noted during the defined observation period. Grade 3 or 4 TRAEs occurred in 87% of patients receiving CaboNivoIpi and 75% of patients receiving CaboNivo. Although there were no DLTs at the highest dose levels using cabozantinib 60 mg daily during the observation period, there were many grade 1 and 2 toxicities attributable to cabozantinib requiring dose holding or dose reduction to cabozantinib 40 mg. There were no grade 5 TRAEs, and immune-related AEs (irAEs) were similar among nivolumab dose levels.
TABLE 3.
Adverse Events
TABLE 4.
Summary of Adverse Events
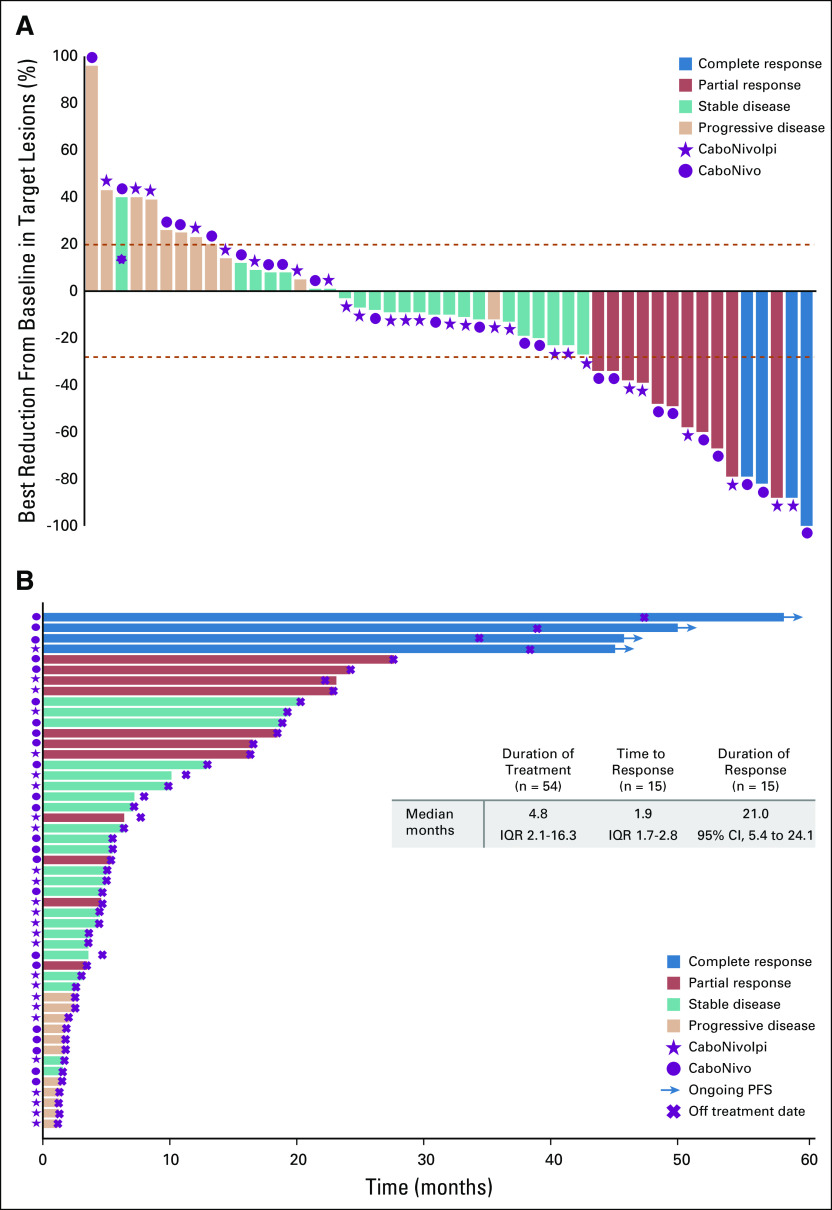
In the 49 patients evaluable for tumor response, the confirmed ORR was 30.6% (15 of 49 patients; 95% CI, 18.3% to 45.4%), and four patients (8.2%) had a CR (Fig 1A and Data Supplement). One patient (included as a responder) had pseudoprogression in the liver (Data Supplement). The DCR was 77.6% (38 of 49 patients; 95% CI, 63.4% to 88.2%), and the median DoR was 21.0 months (95% CI, 5.4 to 24.1 months; Fig 1B). For all patients (N = 54), the median PFS was 5.1 months (95% CI, 3.5 to 6.9 months), and the median OS was 12.6 months (95% CI, 6.9 to 18.8 months; Figs 2A and 2B). Among responders (n = 15), the median OS and PFS are shown in Figures 2C and 2D. Efficacy and follow-up for the CaboNivo and CaboNivoIpi groups are reported in Table 5 and the Data Supplement.
FIG 1.
Clinical activity of cabozantinib and nivolumab (CaboNivo) and cabozantinib, nivolumab, and ipilimumab (CaboNivoIpi). (A) Plot of confirmed tumor regression from baseline as measured by RECIST in all evaluable patients (n = 49). Upper dotted line represents progression at 20%; lower dotted line represents the RECIST boundary for complete response or partial response at 30%. (*) Patient with 40% increase in longest diameter of targeted lung lesion with cavitation. The protocol prespecified that patients with lung cavitary lesions who are experiencing clinical benefit may be allowed to stay on therapy until they experience disease progression based on noncavitary lung lesions. (B) Time to response, duration of treatment, and duration of response to CaboNivo and CaboNivoIpi (16 confirmed responses as of data cutoff). Numbers represent duration of response in months. IQR, interquartile range; PFS, progression-free survival.
FIG 2.
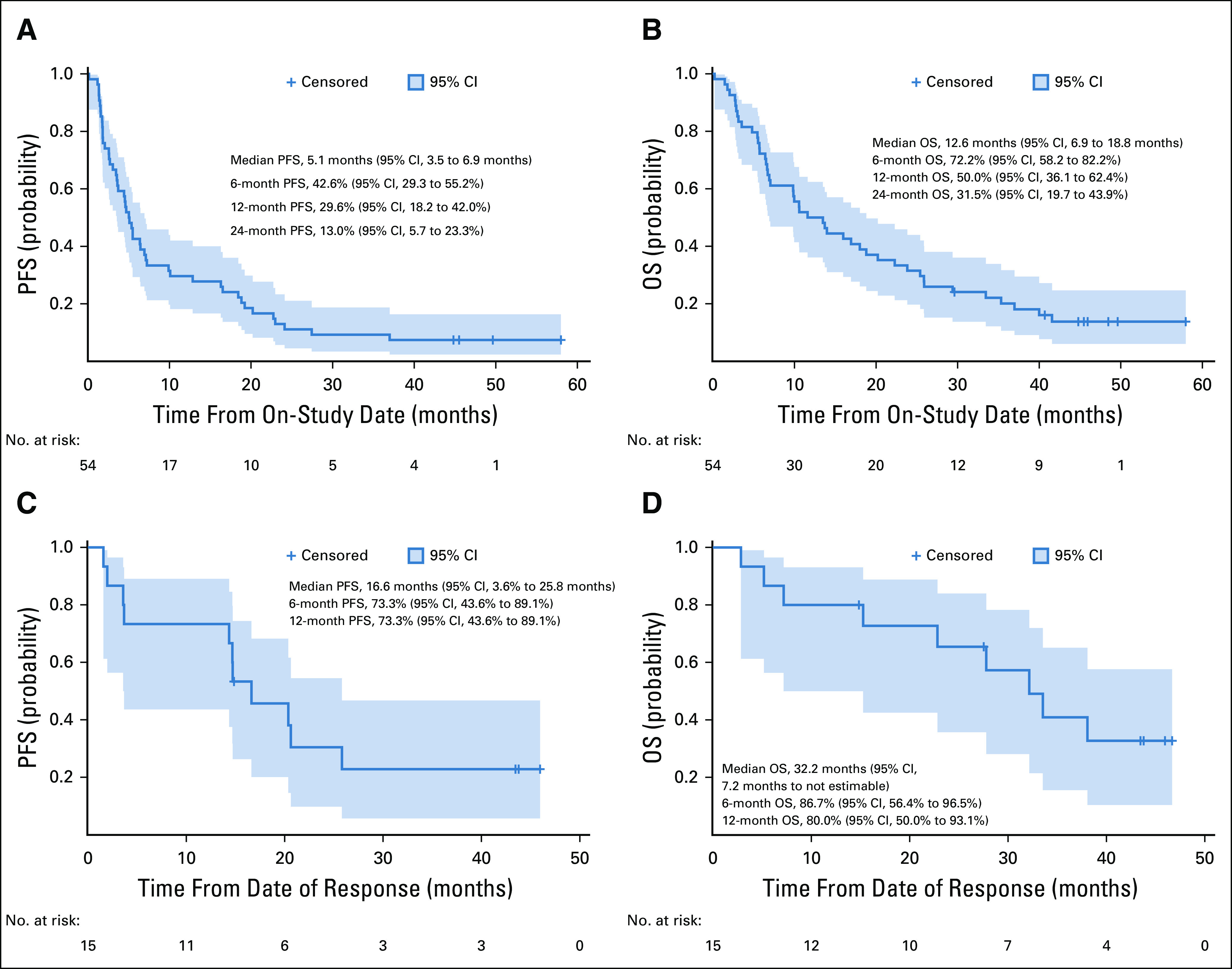
Kaplan-Meier estimates of (A) progression-free survival (PFS) and (B) overall survival (OS) for overall study population (N = 54). Kaplan-Meier estimates of (C) PFS and (D) OS for responding patients (complete or partial response; n = 15). Vertical lines show censored events.
TABLE 5.
Clinical Activity: Confirmed Best Objective Response
Among patients with mUC (15 [28%] of 54 patients; seven treated with CaboNivo and eight treated with CaboNivoIpi), the ORR for evaluable patients was 38.5% (five of 13 patients; 95% CI, 13.9% to 68.4%), and three patients (23.1%) had a CR (Table 5 and Data Supplement). Among responders with mUC (n = 5), the 24-month DoR probability was 80.0% (95% CI, 20.4% to 96.9%). Median DoR was not reached at the time of analysis. For patients with mUC (n = 15), median PFS was 12.8 months (95% CI, 1.8 to 24.1 months); median OS was 25.4 months (95% CI, 5.7 to 41.6 months). One (11.1%; 95% CI, 0.3% to 48.3%) of nine patients with CRPC achieved a PR, and seven patients (77.8%; 95% CI, 40.0% to 97.2%) had SD (Table 5 and Data Supplement). No objective responses were observed in patients with mGCT (Table 5 and Data Supplement). Clinical activity was also observed in patients with urachal adenocarcinoma; one had a PR lasting 16.2 months, and three patients had SD lasting 18.3, 16.2, and 5.2 months, including one patient with reduced ascites. Patients with penile squamous cell carcinoma also demonstrated clinical benefit (Table 5).
Five CaboNivo patients were challenged with ipilimumab at PD, and four CaboNivoIpi patients were rechallenged with ipilimumab at PD. There were no objective responses in this exploratory cohort. Additional data on outcomes for all patients in this exploratory cohort, including patients in the expansion cohorts, will be reported separately. A baseline CTC count of < 5, compared with a CTC count of ≥ 5, was associated with longer median OS in patients with EpCAM-positive cells, EpCAM- and MET-positive cells, and EpCAM- and CXCR4-positive cells (Data Supplement).
DISCUSSION
This phase I study demonstrated that CaboNivo and CaboNivoIpi toxicities can be managed in patients with advanced GU tumors. The safety profiles were largely similar between CaboNivo and CaboNivoIpi, with a slightly higher incidence of some grade 3 or 4 clinical and laboratory TRAEs with CaboNivoIpi. The longer duration of treatment for CaboNivo than for CaboNivoIpi (6.36 v 3.7 months, respectively) may have led to the higher TRAEs observed in some cases. The grade 3 or 4 TRAE rates for CaboNivo (75%) and CaboNivoIpi (87%) were higher than those previously reported in other studies of nivolumab plus ipilimumab27,39 in part as a result of the longer follow-up in our study and the addition of cabozantinib. Although cabozantinib led to more grade 3 or 4 TRAEs, including hypertension, neutropenia, lymphopenia, amylase elevation, and hypophosphatemia, than previously reported in trials with ICIs,27,39 these were manageable. irAEs, including hepatitis and colitis, were similar to those previously reported with nivolumab monotherapy and nivolumab plus ipilimumab and were higher with CaboNivoIpi (30%) than with CaboNivo (13%).
Overlapping toxicities with the use of TKIs and ICIs included thyroid dysfunction, diarrhea, and elevated liver enzymes. The TRAEs of hypothyroidism (32% of patients) and hyperthyroidism (11% of patients) were commonly attributed to all study agents because it was difficult to distinguish between a TKI-caused TRAE and an irAE. Diarrhea was easier to attribute to either a TKI or ICI. Cabozantinib-induced diarrhea occurred as small, frequent stools associated with meals and was generally controlled by holding doses for 5-7 days, dose reduction if recurrent, and antidiarrheal agents. Immune-related diarrhea or colitis tended to be more liquid, was associated with cramping and larger volumes, persisted despite dose holding of all agents or treatment with antidiarrheal agents, and required high-dose corticosteroids. Elevated liver enzymes (ALT and AST) were a common TRAE, and often, both AST and ALT were concurrently elevated. Grade 3 or 4 liver enzyme elevation occurred in two patients treated with CaboNivo and two patients treated with CaboNivoIpi. Immune-related hepatitis requiring high-dose corticosteroids occurred in four patients treated with CaboNivoIpi and in no patients treated with CaboNivo. Overall, hepatic toxicities were manageable with judicious dose holds, reductions, and/or conservative therapy.
Cabozantinib 60 mg/d led to higher rates of clinical TRAEs of all grades, including fatigue, diarrhea, anorexia, weight loss, nausea, vomiting, mucositis, and dehydration. Although the study did not have any DLTs, the RP2Ds were cabozantinib 40 mg/d plus nivolumab 3 mg/kg for the doublet and cabozantinib 40 mg/d, nivolumab 3 mg/kg, and ipilimumab 1 mg/kg for the triplet, based on better clinical tolerability and similar efficacy of cabozantinib at 40 mg/d compared with 60 mg/d.
The study had a long median follow-up time of nearly 45 months, a promising ORR of 30.6%, and a median OS of 12.6 months in a heterogeneous group of patients with metastatic GU tumors, including tumor types with poor prognosis such as renal medullary carcinoma, small-cell bladder cancer, and primitive neuroectodermal tumor. Among the 15 responders, the median OS was 32.2 months.
In patients with mUC, the efficacy was higher than previously reported for single-agent ICIs (15%-20%)24 or monotherapy with cabozantinib (19%),17 with an ORR of 38.5%, DCR of 92.3%, median PFS of 12.8 months, and median OS of 25.4 months. Other smaller tumor cohorts that showed promising responses included clear cell and sarcomatoid RCC, pure squamous cell carcinoma of the bladder, and urethral squamous cell carcinoma. Given these promising findings, expansion cohorts were added to the study.
Although ORR was numerically higher in the CaboNivo group than in the CaboNivoIpi group (39.1% v 26.9%, respectively), patients treated in the triplet group had more aggressive tumors and rarer histologies, such as renal medullary carcinoma, primitive neuroectodermal tumor, Sertoli cell tumor, small-cell bladder/upper tract tumors.
No responses were seen in patients who were challenged or rechallenged with ipilimumab at PD. Three recent studies evaluating similar challenge or rechallenge strategies reported modest efficacy in RCC.40-42
Our exploratory analysis demonstrated that baseline CTC levels of less than five cells were associated with prolonged OS (Data Supplement). However, changes in CTCs during treatment were not associated with treatment response or outcome. To explore the role of the cabozantinib target MET in the current trial, we looked at both total EpCAM-positive CTCs and the subset of CTCs expressing MET and found that a baseline CTC count of less than five, compared with a CTC count of ≥ 5, was associated with longer median OS for patients with EpCAM-positive, EpCAM- and MET-positive, and EpCAM- and CXCR4-positive cells, demonstrating that MET and CXCR4 expression in CTCs at baseline is associated with poorer survival.
Our study is limited by the tumor heterogeneity and small sample size in each group. Correlative analysis should be interpreted cautiously.
In conclusion, this phase I study of CaboNivo and CaboNivoIpi in metastatic GU tumors demonstrated tolerable AEs, including manageable overlapping toxicities. The combinations of CaboNivo and CaboNivoIpi seem to have high clinical activity relative to the reported monotherapy with each agent. In fact, the broad applicability of both combinations makes them attractive treatment options for many solid tumors in which TKIs and ICIs have already demonstrated activity. The promising activity seen in this phase I study has led to additional expansion cohorts within this study, including cohorts for urothelial carcinoma, RCC, and other rare GU tumors with no standard treatment options, and has also led to larger trials in GU tumors, including CheckMate 9ER (ClinicalTrials.gov identifier: NCT03141177), a randomized phase III trial of CaboNivo versus sunitinib in the first-line treatment of mRCC; PDIGREE (ClinicalTrials.gov identifier: NCT03793166), an adaptive phase III trial of CaboNivoIpi in untreated mRCC; COSMIC-313 (ClinicalTrials.gov identifier: NCT03937219), a phase III trial of CaboNivoIpi versus NivoIpi plus placebo in mRCC; and the Alliance ICONIC study (ClinicalTrials.gov identifier: NCT03866382) of CaboNivoIpi for rare GU tumors. Several other trials are testing CaboNivo in non–clear cell RCC (ClinicalTrials.gov identifier: NCT03635892), carcinoid tumors (ClinicalTrials.gov identifier: NCT04197310), metastatic triple-negative breast cancer (ClinicalTrials.gov identifier: NCT03316586), locally advanced hepatocellular carcinoma (ClinicalTrials.gov identifier: NCT03316586), advanced endometrial cancer (ClinicalTrials.gov identifier: NCT03367741), recurrent uterine carcinosarcoma (ClinicalTrials.gov identifier: NCT04149275), poorly differentiated neuroendocrine tumors (ClinicalTrials.gov identifier: NCT04079712), and non–small-cell lung cancer (ClinicalTrials.gov identifiers: NCT04310007 and NCT03468985). A study of CaboNivoIpi in unresectable advanced melanoma (ClinicalTrials.gov identifier: NCT04091750) is also underway.
PRIOR PRESENTATION
Presented, in part, at the 54th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018; the 2018 American Society of Clinical Oncology Genitourinary Cancers Symposium, San Francisco, CA, February 8-10, 2018; and the 2017 European Society for Medical Oncology Congress, Madrid, Spain, September 8-12, 2017.
SUPPORT
Supported by the National Cancer Institute’s Intramural Research Program (Grant No. ZIA BC 011351, 011594); the Cancer Therapy Evaluation Program of the National Institutes of Health, Bethesda, MD; and the Experimental Therapeutics Clinical Trials Network UM1 award.
DISCLAIMER
Patients have granted consent to the authors for use of photographic and radiologic images used in this publication.
CLINICAL TRIAL INFORMATION
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI https://doi.org/10.1200/JCO.20.01652.
AUTHOR CONTRIBUTIONS
Conception and design: Andrea B. Apolo, William D. Figg, Howard Streicher, John J. Wright, William L. Dahut, Donald P. Bottaro
Administrative support: Andrea B. Apolo, James L. Gulley, Howard Streicher, Primo N. Lara Jr
Provision of study materials or patients: Andrea B. Apolo, Paul Monk, Piyush K. Agarwal, Maria J. Merino, Primo N. Lara Jr, Biren Saraiya, Mark N. Stein, Amir Mortazavi
Collection and assembly of data: Andrea B. Apolo, Rosa Nadal, Daniel M. Girardi, Lisa Ley, Lisa M. Cordes, Olena Sierra-Ortiz, Jacqueline Cadena, Carlos Diaz, Marissa Malleck, Nicole N. Davarpanah, Rene Costello, Jane B. Trepel, Mohammad Bagheri, William D. Figg, Piyush K. Agarwal, Heather J. Chalfin, Jennifer Jones, Maria J. Merino, Howard L. Parnes, Donald P. Bottaro, Primo N. Lara Jr, Biren Saraiya, Sumanta K. Pal, Mark N. Stein, Amir Mortazavi
Data analysis and interpretation: Andrea B. Apolo, Rosa Nadal, Daniel M. Girardi, Seth M. Steinberg, Nicole N. Davarpanah, Jane B. Trepel, Min-Jung Lee, Paul Monk, Maria J. Merino, James L. Gulley, Vladimir Valera, Jennifer Jones, Yangmin M. Ning, Howard L. Parnes, William L. Dahut, Donald P. Bottaro, Primo N. Lara Jr, Biren Saraiya, Mark N. Stein, Amir Mortazavi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I Study of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Scot A. Niglio
Stock and Other Ownership Interests: Regeneron, Gilead Sciences
Other Relationship: STEMCELL Technologies (I)
Nicole N. Davarpanah
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Travel, Accommodations, Expenses: Genentech
Jane B. Trepel
Research Funding: Syndax Pharmaceuticals (Inst), EpicentRX (Inst), AstraZeneca (Inst)
Paul Monk
Honoraria: Sanofi-Aventis
Consulting or Advisory Role: Dendreon
Speakers' Bureau: Janssen
William D. Figg
Research Funding: Celgene (Inst), Astellas Pharma (Inst), Nerviano Medical Sciences (Inst), Pfizer (Inst), NovaRX (Inst), TRACON Pharmaceuticals (Inst), Biocompatibles (Inst)
James L. Gulley
Research Funding: EMD Serono (Inst), Bavarian Nordic (Inst), Astellas Medivation (Inst), Pfizer (Inst), NantBioScience (Inst), Bristol Myers Squibb (Inst), Merck (Inst)
Piyush K. Agarwal
Employment: Pfizer (I)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune
Jennifer Jones
Patents, Royalties, Other Intellectual Property: National Cancer Institute intellectual property (IP) relating to liquid biopsies, including extracellular vesicles and viral detection and analysis; Stanford University IP relating to TIM family proteins
Howard L. Parnes
Stock and Other Ownership Interests: Illumina, Health Care Select SPDR, Gilead Sciences, Roper, Intuitive Surgical, Editas Medicine, Novartis, Roche, Intellia Therapeutics, Schlumberger, Iqvia Holdings
Donald P. Bottaro
Patents, Royalties, Other Intellectual Property: Bottaro DP, Petryshyn R. US Patent No. 6,326,466; December 4, 2001: Double Stranded RNA Dependent Protein Kinase Derived Peptides to Promote Proliferation of Cells and Tissues in a Controlled Manner. Related International Publication No. WO/1998/004717; Chan AML, Rubin JS, Bottaro DP, Aaronson SA. US Patent No. 6,566,098; May 20, 2003: DNA Encoding Truncated Hepatocyte Growth Factor Variants. Related International Publication No. WO/1992/005184; Bottaro DP, Soriano JV, Atabey SN, Breckenridge DE, Gao Y, Yao Z-J, Burke TR Jr. US Patent No. 7,132,392; November 7, 2006: Inhibition of Cell Motility and Angiogenesis by Inhibitors of Grb2-SH2-Domain. Related International Publications No. WO/2001/028577 and No. WO/2008/036565; Chan AML, Rubin JS, Bottaro DP, Aaronson SA, Stahl SJ, Wingfield PT, Cioce V. US Patent No. 7,605,127; October 20, 2009: Truncated Hepatocyte Growth Factor Variant Protein HGF/NK2. Related International Publication No. WO/1996/040914; Bottaro DP, Giubellino A, Atabey N, Soriano JV, Breckenridge DE, Burke TR Jr. US Patent No. 7,871,981; January 18, 2011: Inhibition of Cell Motility, Angiogenesis, and Metastasis. Related International Publication No. WO/2001/028577; Bottaro DP, Athauda G, Burgess TL. US Patent No. 7,964,365; June 21, 2011: Methods for Diagnosing and Monitoring the Progression of Cancer. Related International Publication No. WO/2007/056523; Bottaro DP, Athauda G, Burgess TL. US Patent No. 8,304,199; November 6, 2012: Methods for Diagnosing and Monitoring the Progression of Cancer by Measuring Soluble c-Met Ectodomain; Bottaro DP, Peach M, Nicklaus M, Tan N. US Patent No. 8,569,360; October 29, 2013: Compositions and Methods for Inhibition of Hepatocyte Growth Factor Receptor c-Met Signaling. Related International Publications No. WO/2009/124024 and WO/2009/124013; Bottaro DP, Athauda G, Burgess TL. US Patent No. 8,617,831; December 31, 2013: Methods for Diagnosing and Monitoring the Progression of Cancer by Measuring Soluble c-Met Ectodomain; Bottaro DP, Peach M, Nicklaus M, Burke TR Jr, Athauda G, Choyke S, Giubellino A, Tan N, Shi Z-D. US Patent No. 8,754,081; June 17, 2014: Compositions and Methods for Inhibition of Hepatocyte Growth Factor Receptor c-Met Signaling. Related International Publication No. WO/124013; Bottaro DP, Cecchi F. US Patent No. 9,550,818, January 24, 2017: Methods for Use of Vascular Endothelial Growth Factor Antagonists. Related International Publication No. WO/2013/163606; Bottaro DP, Cecchi F. US Patent No. 10,035,833, July 31, 2018: Vascular Endothelial Growth Factor Antagonists and Methods of Making.
Primo N. Lara Jr
Consulting or Advisory Role: Janssen
Research Funding: Aragon Pharmaceuticals (Inst), Janssen Biotech (Inst), TRACON Pharmaceuticals (Inst), Merck (Inst), Pharmacyclics (Inst), Incyte (Inst), Taiho Pharmaceutical (Inst)
Biren Saraiya
Honoraria: Sanofi, Eisai
Sumanta K. Pal
Consulting or Advisory Role: Pfizer, Novartis, Aveo, Myriad Pharmaceuticals, Genentech, Exelixis, Bristol Myers Squibb, Astellas Pharma, Ipsen, Eisai
Mark N. Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Medivation/Astellas (Inst), Advaxis (Inst), Suzhou Kintor Pharmaceuticals (Inst), Harpoon (Inst), Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Eli Lilly (Inst), Nektar (Inst), Seattle Genetics (Inst), Xencor (Inst), Tmunity (Inst), Exelixis (Inst)
Amir Mortazavi
Honoraria: Motive Medical Intelligence
Consulting or Advisory Role: Seattle Genetics, Debiopharm Group, Pfizer
Research Funding: Acerta Pharma (Inst), Genentech (Inst), Merck (Inst), Novartis (Inst), Seattle Genetics (Inst), Mirati Therapeutics (Inst), Bristol Myers Squibb (Inst), Roche (Inst), Astellas Pharma (Inst), Debiopharm Group (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg JE Hoffman-Censits J Powles T, et al. : Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 387:1909-1920, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P Retz M Siefker-Radtke A, et al. : Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol 18:312-322, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Bellmunt J de Wit R Vaughn DJ, et al. : Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015-1026, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apolo AB Infante JR Balmanoukian A, et al. : Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: Results from a multicenter, phase Ib study. J Clin Oncol 35:2117-2124, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T O’Donnell PH Massard C, et al. : Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 3:e172411, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Velasco G Bex A Albiges L, et al. : Sequencing and combination of systemic therapy in metastatic renal cell carcinoma. Eur Urol Oncol 2:505-514, 2019 [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.1016/j.clgc.2020.02.005. Hird AE, Magee DE, Bhindi B, et al: A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Clin Genitourin Cancer . [epub ahead of print on March 6, 2020] [DOI] [PubMed] [Google Scholar]
- 9.de Bono J Mateo J Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Nadal R, Bellmunt J: Management of metastatic bladder cancer. Cancer Treat Rev 76:10-21, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Yakes FM Chen J Tan J, et al. : Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10:2298-2308, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Mazzola CR, Chin J: Targeting the VEGF pathway in metastatic bladder cancer. Expert Opin Investig Drugs 24:913-927, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Lee RJ, Smith MR: Cabozantinib and prostate cancer: Inhibiting seed and disrupting soil? Clin Cancer Res 20:525-527, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottaro DP, Liotta LA: Cancer: Out of air is not out of action. Nature 423:593-595, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Shojaei F Lee JH Simmons BH, et al. : HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res 70:10090-10100, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Sennino B Ishiguro-Oonuma T Wei Y, et al. : Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2:270-287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apolo AB Nadal R Tomita Y, et al. : Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: An open-label, single-centre, phase 2 trial. Lancet Oncol 21:1099-1109, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choueiri TK Escudier B Powles T, et al. : Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17:917-927, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Sonpavde GP Pond GR Fizazi K, et al. : Cabozantinib for progressive metastatic castration-resistant prostate cancer following docetaxel: Combined analysis of two phase 3 trials. Eur Urol Oncol 10.1016/j.euo.2018.11.006 [epub ahead of print on November 30, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beer TM Kwon ED Drake CG, et al. : Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol 35:40-47, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Necchi A Giannatempo P Raggi D, et al. : An open-label randomized phase 2 study of durvalumab alone or in combination with tremelimumab in patients with advanced germ cell tumors (APACHE): Results from the first planned interim analysis. Eur Urol 75:201-203, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Brahmer JR Drake CG Wollner I, et al. : Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28:3167-3175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer RJ Escudier B McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P Callahan MK Bono P, et al. : Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 17:1590-1598, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fellner C: Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. P T 37:503-530, 2012 [PMC free article] [PubMed] [Google Scholar]
- 26.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252-264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motzer RJ Tannir NM McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma P Siefker-Radtke A de Braud F, et al. : Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol 37:1608-1616, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwilas AR Donahue RN Tsang KY, et al. : Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron 2:e677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirkx AE oude Egbrink MG Castermans K, et al. : Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J 20:621-630, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Yasuda S Sho M Yamato I, et al. : Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 172:500-506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motzer RJ Penkov K Haanen J, et al. : Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103-1115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rini BI Powles T Atkins MB, et al. : Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 393:2404-2415, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Rini BI Plimack ER Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Agarwal N Loriot Y McGregor B, et al. : Cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): Results of cohort 6 of the COSMIC-021 study. J Clin Oncol 38, 2020. (suppl 6; abstr 139) [Google Scholar]
- 36.Eisenhauer EA Therasse P Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Skolnik JM Barrett JS Jayaraman B, et al. : Shortening the timeline of pediatric phase I trials: The rolling six design. J Clin Oncol 26:190-195, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Apolo AB Karzai FH Trepel JB, et al. : A phase II clinical trial of TRC105 (anti-endoglin antibody) in adults with advanced/metastatic urothelial carcinoma. Clin Genitourin Cancer 15:77-85, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolchok JD Chiarion-Sileni V Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345-1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKay R Xie W McGregor B, et al. : Optimized management of nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma (RCC): A response-based phase II study (OMNIVORE). J Clin Oncol 38, 2020. (suppl 15; abstr 5005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkins M Jegede O Haas N, et al. : Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (pts) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). J Clin Oncol 38, 2020. (suppl 15; abstr 5006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gershenson D Miller A Brady W, et al. : A randomized phase II/III study to assess the efficacy of trametinib in patients with recurrent or progressive low-grade serous ovarian or peritoneal cancer. Ann Oncol 30:v851-v934, 2019. (suppl 5) [Google Scholar]